Translate this page into:
A detailed review of immunotherapeutics with a special emphasis on hybridoma technology

-
Received: ,
Accepted: ,
How to cite this article: Vaghela AR, Ganatra TH. A detailed review of immunotherapeutics with a special emphasis on hybridoma technology. Am J Biopharm Pharm Sci. 2024;4:2. doi: 10.25259/AJBPS_13_2023
Abstract
The paper offers a thorough analysis of immunotherapeutics with a focus on hybridomas. It describes how focused and precise treatments for a variety of illnesses, such as cancer, autoimmune disorders, and infectious diseases, have been made possible by immunotherapeutics, which are based on antibody and hybridoma technology. The main therapeutics produced by this method are monoclonal antibodies (mAbs). The article describes the hybridoma technology process, in which a heterogeneous population of cells that produce unique mAbs are created by combining immortalized myeloma cells with B lymphocytes. To isolate and create drug formulations, the hybridoma cells that produce the desired antibodies are chosen and grown in large numbers. In the article, successful uses of immunotherapeutics based on antibody and hybridoma technology are highlighted. Hybridoma technology used in treatment of autoimmune conditions, viral infections and cancer. The potential of mAbs to increase the range of available treatments is also covered. The page also describes the distinction between monoclonal and polyclonal antibodies, how they are made, and the different uses of hybridoma technology in research, diagnostics, therapy, vaccine development, and fundamental immunology investigations. The importance of immunotherapeutics based on antibody and hybridoma technologies in revolutionizing the treatment environment and creating new opportunities for customized and targeted therapies is emphasized as it draws to a close.
Keywords
Immunotherapeutics
Hybridoma
Immunomodulators
Monoclonal antibodies
INTRODUCTION
By providing focused and extremely specific treatments for a wide range of ailments, including cancer, autoimmune disorders, and infectious diseases, immunotherapeutics based on antibody and hybridoma technologies have completely changed the practice of medicine. By harnessing the immune system’s strength and fusing it with cutting-edge biotechnology, this method creates monoclonal antibodies (mAbs), which are therapeutic agents that mAbs are molecules created in a laboratory that resembles the immune system’s naturally occurring antibodies. They are made with extreme accuracy to attach to the particular antigens, such as proteins on the surface of cancer cells or pathogens. mAbs can have a variety of therapeutic effects thanks to this tailored binding, such as preventing the function of dangerous compounds, delivering medications or toxins to particular cells, or inciting the immune system to attack sick cells. With the hybridoma technique, mAbs are first developed. A stable cell line that can perpetually produce a particular antibody is produced via hybridomas, which are made by merging immortalized myeloma cells with antibody-producing B lymphocytes from an immunized animal. Through this fusion process, a heterogeneous population of hybridoma cells is created, each of which produces a distinct monoclonal antibody. A screening procedure is used to find and choose the hybridoma cells that produce antibodies with the necessary specificity and functionality after they have been established. To manufacture huge amounts of mAbs, these chosen hybridoma cells are subsequently grown up and cultivated. The antibodies can be isolated and produced into medicinal formulations, either on their own or in combination with other compounds. In numerous clinical applications, immunotherapeutics based on antibody and hybridoma technologies have shown to be incredibly successful and well-tolerated. For instance, a number of mAbs, including rituximab for specific types of lymphomas and trastuzumab for human epidermal growth factor receptor 2-positive breast cancer, have been approved for the treatment of cancer. In addition, mAbs have demonstrated potential in the treatment of viral infections like COVID-19 and autoimmune illnesses, including psoriasis and rheumatoid arthritis. The creation and application of immunotherapeutics based on antibody and hybridoma technology have altered the treatment environment and opened up new avenues for targeted and personalized therapy. The possible uses of mAbs are continually being expanded thanks to ongoing study and developments in the field, opening the door to creative and efficient cures for a variety of ailments.
INTRODUCTION OF IMMUNOTHERAPEUTICS
Immunotherapeutic is an agent which either activates immune response or suppress immune response. The body’s natural defenses against a variety of ailments, including cancer, autoimmune disorders, and infectious diseases, are strengthened by these cutting-edge treatments, which employ a variety of techniques, including mAbs, immune checkpoint inhibitors, cancer vaccines, and adoptive cell therapies. Immunotherapeutics have the ability to provide highly focused and personalized interventions that are frequently associated with less adverse effects than conventional therapies because they target certain molecules or cells implicated in the progression of the disease. Immunotherapeutics have advanced to the forefront of clinical practice and medical research thanks to their astounding effectiveness and capacity to elicit long-lasting responses, giving patients fresh hope and revolutionizing the field of modern healthcare.
INTRODUCTION OF ANTIBODY
Antibodies, also known as immunoglobulins, are crucial components of the immune system, playing a pivotal role in defending the body against invading pathogens. These specialized proteins, produced by B-cells, are designed to recognize and bind to specific antigens, which are molecules present on the surface of pathogens or abnormal cells. Antibodies possess a Y-shaped structure, with two arms that can bind to antigens and a tail that interacts with other components of the immune system. By binding to antigens, antibodies can neutralize pathogens directly, mark them for destruction by other immune cells, or activate complement proteins to enhance immune responses.
Antibody structure
The Y-shaped structure of an antibody is composed of four polypeptide subunits. There are two identical light and heavy chains in each subunit.[1] Each heavy chain’s N-terminus joins with a light chain to produce an antigen-binding domain. The two antigen-binding domains that make up the “Y”’s arms are present. “Fragment antigen-binding” domains are what they are called. The interaction with the effector cells is facilitated by the formation of the “fragment crystallization” domain at the C-terminus of the heavy chains. Disulfide and non-covalent bonds hold the four polypeptide subunits together. A variable area and three consistent sections can be found in the heavy chains of antibodies. The two antigen-binding sites are unique to each antibody and are identical in all antibodies.
Immunoglobulin G (Ig G) is a type of antibody. Representing approximately 75% of serum antibodies in humans, IgG is the most common type of antibody found in blood circulation. IgG molecules are created and released by plasma B-cells. Each IgG antibody has two paratopes.
Immunoglobulin A (IgA, also referred to as IgA in its secretory form) IgA is produced in larger amounts in conjunction with mucosal membranes than all other types of antibodies combined.
Immunoglobulin M (IgM) is one of several antibody isotypes (also known as immunoglobulins) that vertebrates make. The biggest antibody, IgM, is also the first to show up when the body reacts to an antigen for the 1st time.
Immunoglobulin E (IgE) is an antibody subtype (or immunoglobulin [Ig] “isotype”) that has only been identified in animals. Plasma cells manufacture IgE. IgE monomers are made up of two heavy chains (the chain) and two light chains. The chain has four constant domains that resemble those found in Ig (C1–C4).
Immunoglobulin D (IgD) is an antibody isotype that often coexpresses with the cell surface antibody IgM and accounts for around 1% of the proteins in the plasma membranes of immature B-lymphocytes [Figure 1].
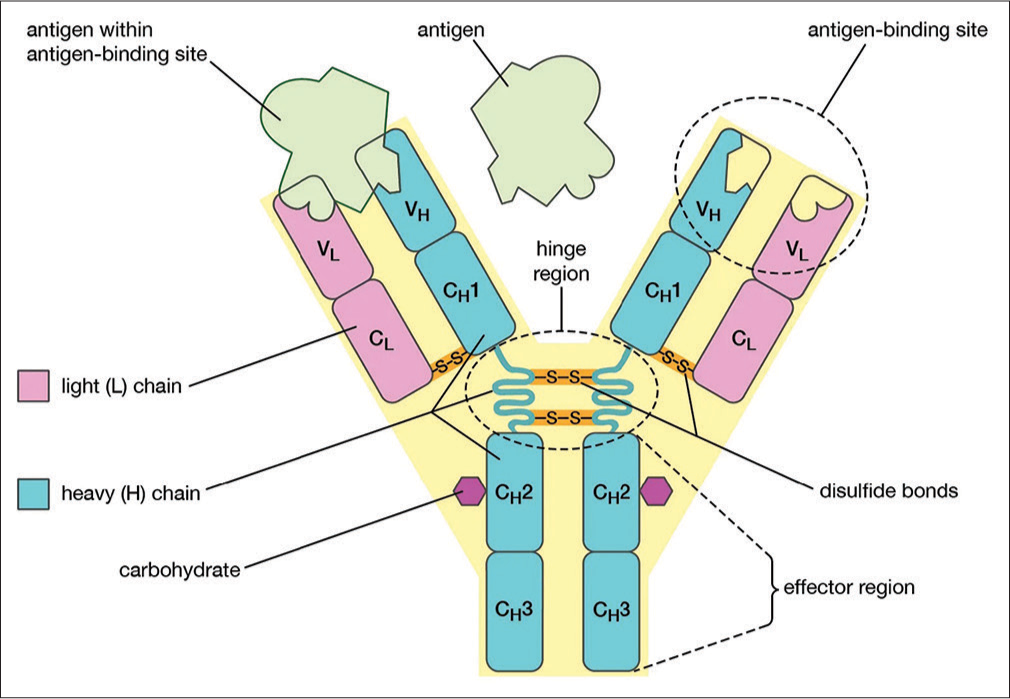
- Antibody structure. The base of the antibody includes constant domains (C). VH – heavy chain variable domain, VL- light chain variable domain, CH – heavy chain constant domain, CL – light chain constant domain.
TYPES OF IMMUNOTHERAPEUTIC
mAbs
These antibodies are created in laboratories and are intended to bind to particular antigens on target cells or molecules. They can deliver medications directly to the target, mark cells for apoptosis, or disrupt signaling pathways. Cancer, autoimmune disorders, and infectious diseases have all been successfully treated with mAbs.
Checkpoint inhibitors
Immune checkpoint inhibitors aim immune cells’ or cancer cells’ regulatory proteins. They aid in releasing the immune system’s capacity to identify and combat cancer cells by blocking these checkpoints. Some cancers, such as melanoma and lung cancer, have responded very well to treatment with checkpoint inhibitors.
Adoptive cell therapies
Adoptive cell therapies include removing and modifying a patient’s immune cells, including T-cells or natural killer (NK) cells, to improve their capacity to identify and eradicate cancer cells. The patient is then given these modified cells again to launch a powerful immunological attack against the illness.
Cancer vaccines
The goal of cancer vaccines is to activate the immune system so that it will recognize and attack cancer cells.[2] They may be made up of immune-stimulating chemicals or tumor-specific antigens that trigger an immune response against cancer cells. Certain cancer vaccines have demonstrated promising effects when used to treat particular cancers.[3]
Cytokines
Cytokines are immune system-regulating proteins. They can be employed therapeutically to increase the immune system’s activity against infectious or malignant disorders. Interferons and interleukins are a couple of examples.[4]
Chimeric antigen receptor (CAR) T
Cell treatment is genetically altering a patient’s T-cells to express certain receptors (CARs) that are able to recognize and destroy cancer cells. When used to treat specific types of blood malignancies, CAR-T cell therapy has been shown to be quite effective.[5]
DIFFERENCE BETWEEN MONOCLONAL AND POLYCLONAL ANTIBODY
The fundamental distinction between mAbs and polyclonal antibodies is the technology used in their synthesis as well as their level of specificity. A single target antigen is recognized with high specificity by mAbs, which are produced from a single clone of B-cells. A population of antibodies with a high degree of uniformity is created in the laboratory using genetic engineering techniques. Polyclonal antibodies, on the other hand, are produced by an organism’s immune system in response to an antigen. They are an amalgam of antibodies made by various B-cell clones, each of which recognizes a distinct epitope on the target antigen. The immunization of an animal, followed by the collection of serum or plasma, is how polyclonal antibodies are made.[6] Polyclonal antibodies give a wider variety, whereas mAbs provide consistent and accurate targeting of binding sites and possibly provide superior protection against various epitopes.
PRODUCTION OF MONOCLONAL ANTIBODY
Production of mAb by the hybridoma technology was discovered by George Kohler, Cesar Milstein, and Niles Jerne. For this research, they got the Nobel Prize in 1984.
HYBRIDOMA TECHNOLOGY
Principle
The hybrid cell has the ability to produce antibodies using B-cells (spleen cells). It can divide constantly using a quality obtained from myeloma cells at the same time. The approach makes sure that a lot of antibodies with a single specificity are produced by integrating the appropriate characteristics of both cells. Spleen cell and myeloma cell-specific hybridomas can produce mAbs in artificial media; this process is known as hybridoma technology.[6]
Procedure
mAbs are produced in vast numbers in laboratories using hybridoma technology. mAbs, also referred to as hybridoma cells, are very specific antibodies that come from a single parent cell. Here is a detailed process for using hybridoma technology [Figure 2].[7]

- Hybridoma technology procedure. HAT Medium: hypoxanthine-aminopterin-thymidine medium.
Immunization
The procedure starts by giving a mouse or another suitable animal the target antigen. The creation of antibodies is triggered by the presence of an antigen, which is a substance.[8]
Cell fusion
The next stage is to remove the immunized animal’s spleen cells after an adequate immunological response has been produced. B-cells that produce antibodies are found in the spleen. The myeloma cell type of malignancy, which has an infinite capacity for division, is then united with these cells.[8]
Cell culture and selection
The fused cells, known as hybridomas, are cultivated in a medium that is selective, allowing only hybridoma cells to survive while inhibiting the growth of unfused parent cells. Aminopterin, which suppresses the proliferation of myeloma cells that have not merged and do not make antibodies, is typically present in the selective medium.[8]
Screening
Identification of the hybridoma cells that produce antibodies specific to the target antigen is done by screening. An enzyme-linked immunosorbent assay (ELISA) test or a method comparable to it is commonly used for this. For further propagation, positive hybridoma cells are chosen since they produce the necessary antibodies.[8]
Cloning
The production of a single kind of antibody is ensured by the use of cloning. This entails dividing distinct hybridoma cells and permitting them to develop into distinct colonies, each deriving from a single hybridoma cell. This guarantees that each colony generates unique monoclonal antibody.[8]
Production of antibodies
To create a significant amount of mAbs, the chosen hybridoma cells are cultivated at a bigger scale. Usually, bioreactors are used to cultivate these cells in such specialized containers.[8]
Antibody purification
mAbs are extracted from the cell culture supernatant, which also contains the released antibodies, during the antibody purification process. The antibodies are isolated and purified using a variety of purification methods, including chromatography, filtering, and precipitation.[8]
Characteristics
Purified mAbs are subjected to characterization to ascertain their specificity, affinities, and other features. By doing this, the appropriate quality standards for the antibodies are made sure to be met.[9]
Storage and distribution
The final stage entails storing the mAbs in the proper manner and dispersing them for use in various applications, such as research, diagnostics, or therapeutic use. It is vital to remember that the approach described above is only a general description of the hybridoma technology process. Depending on the specific application and laboratory protocols, there may be specifics and differences.[9]
APPLICATION OF HYBRIDOMA TECHNOLOGY
Research tools
Hybridoma-produced mAbs are useful for doing research. In a variety of biological samples, they can be used to investigate the expression, location, and functionality of particular proteins. In addition, mAbs are employed in procedures including Western blotting, immunohistochemistry, and immunofluorescence.[10]
Diagnostics
mAbs made from hybridomas are frequently employed in diagnostic procedures. Target molecules, such as pathogens, tumor indicators, hormones, or certain cell surface antigens, can be selectively recognized by them and bound to. These antibodies make it possible to create sensitive and accurate diagnostic assays, such as lateral flow assays, ELISAs, and immunohistochemistry-based diagnostic tests.[10]
Therapeutics
The production of mAbs using hybridoma technology has completely changed the therapeutics industry. They are used to treat a variety of illnesses, such as cancer, autoimmune conditions, and infectious diseases. Examples are infliximab for rheumatoid arthritis, trastuzumab for breast cancer, and rituximab for lymphoma.[10]
Vaccine development
The development of vaccines is greatly aided by hybridoma technology. Antigens crucial for immune defense can be recognized and described using mAbs. They contribute to the development and assessment of vaccines by shedding light on immune responses and antigen-antibody interactions.
Basic immunology studies
The creation of mAbs against certain antigens is made possible by hybridoma technology, which makes it easier to understand the fundamental concepts of immunology. It makes it possible to investigate immune system elements, including cytokines, receptors, and immune cell markers, which improves our comprehension of immune responses and their mechanisms.
Cancer research
Cancer research has greatly benefited from the use of mAbs generated from hybridomas. They are applied to recognize and target certain cancer cells or antigens linked to tumors. Through antibody-based therapies like immunotherapy, mAbs can help in cancer diagnosis, staging, and monitoring as well as act as therapeutic agents.[11]
Applications in veterinary medicine
Hybridoma technology has uses in veterinary medicine. Animals with cancer and infectious disorders, among others, can be treated using mAbs. They also make studying easier.[11]
TYPES OF MONOCLONAL ANTIBODY
Mouse
Produce human-anti-mouse antibodies hypersensitivity reaction-III using 100% mouse protein.
Human
100% human protein combined with human; no immune responses, adalimumab, are one example.
Chimeric
33% mouse protein chimeric mouse is a variable part. Constant fraction = decreases in human immune system responses consider basiliximab.[12]
Humanized
Using 5–10% mouse protein to make humanized mouse is a variable part. Human immune system decrease the reaction constant, for instance, bevacizumab.[13]
FUSION PROTEIN
A type of medicinal compounds utilized in immunotherapy is called fusion proteins. Genetic sequences encoding various functional domains from two or more proteins are combined to produce them. Fusion proteins are created specifically for immunotherapeutics to target particular cells or molecules or to improve immune responses by combining the capacities of other proteins. Examples of fusion proteins utilized in immunotherapy include the following:
Immunotoxins
Fusion proteins that combine an antibody or an antibody fragment with a toxin are known as immunotoxins. The toxin component delivers a cytotoxic payload to kill the target cells, while the antibody component binds to a particular target molecule on the surface of cancerous or diseased cells. For the treatment of many malignancies and infectious disorders, immunotoxins have been created.[11]
Bispecific antibodies
Fusion proteins called bispecific antibodies fuse two distinct antigen-binding sites into a single molecule. They can bind to two separate target molecules at once, such as an immune cell receptor and a cancer cell antigen. Bispecific antibodies are thus able to draw immune cells in close proximity to cancer cells, boosting the immune response and enabling the deliberate destruction of cancer cells.[14]
Cytokine fusion proteins
Cytokines are signaling molecules that have a role in controlling the immune system. To increase their specificity and effectiveness, fusion proteins that combine cytokines with antibody fragments or other targeting domains have been created. By targeting cytokines to particular cell types or tumor microenvironments, these fusion proteins can increase their therapeutic efficacy while minimizing systemic negative effects.[14]
CAR T-cell therapy
CAR T-cell therapy is a cutting-edge immunotherapeutic strategy for the treatment of cancer. It entails genetically altering a patient’s T-cells to express a chimeric antigen receptor, which consists of intracellular signaling domains and an antigen-binding domain commonly taken from an antibody. By allowing T lymphocytes to recognize and destroy cancer cells that are overexpressing the target antigen, this fusion protein helps to eradicate tumors.[15]
Fusion proteins for immunomodulation
By combining immune checkpoint inhibitors or costimulatory molecules with targeting domains, fusion proteins can be created to influence immune responses. These fusion proteins have the potential to boost immune activation or block immunological checkpoint pathways, enhancing anti-tumor immune responses and providing significant therapeutic advantages.
Immunotherapeutics that use fusion proteins enable precision targeting, increased efficacy, and better therapeutic results by combining various capabilities into a single molecule. They remain a focus of ongoing research and development in the field of immunotherapy, providing fresh options for the treatment of a variety of illnesses, including cancer and immune-related disorders.
RECOMBINANT CYTOKINES
Recombinant cytokines are proteins created by genetic engineering methods, enabling the mass synthesis of particular cytokines. Signaling molecules called cytokines that are responsible for the control of inflammatory processes, immunological responses, and cell division. Following are few examples of recombinant cytokines and their purposes:[16]
Interleukins (IL)
Interleukins are a class of cytokines that control inflammation and immunological responses.[17]
IL-2
Recombinant T-cells, NK cells, and other immune cells are encouraged to proliferate and become activated by IL-2. It has been applied as an immunotherapy for some cancer types.[17]
Recombinant IL-4
IL-4 encourages naive T-cells to differentiate into helper T-cells, which support the generation of antibodies. It has been researched for its potential in the treatment of allergy and autoimmune illnesses.[16]
IL-6 recombinant
IL-6 contributes to inflammation and the acute-phase response. Its medicinal potential in treating diseases, including rheumatoid arthritis and some malignancies has been researched.[17]
Tumor necrosis factors (TNF)
TNF plays a role in inflammation and immunological reactions. Recombinant TNF-alpha: TNF-alpha is a pro-inflammatory cytokine involved in cell death, inflammation, and immunological responses. Recombinant TNF-alpha has been applied to the treatment of melanoma and sarcoma, among other malignancies.
Interferons (IFN)
IFNs play a role in controlling the immune system and antiviral defenses.[17]
IFN-alpha
A recombinant protein possesses antiviral and immunomodulatory properties. It has been used to treat various cancers, including leukemia and melanoma, as well as viral infections including hepatitis B and C.
Recombinant IFN-gamma
IFN-gamma is a protein that plays a role in immune responses by activating macrophages and increasing antigen presentation. It has been investigated for its potential to treat disease and cancer.[17]
Colony-stimulating factors (CSF)
CSFs control how hematopoietic cells develop and differentiate.
Granulocyte-CSF (G-CSF) Recombinant White blood cells called neutrophils are produced and function more effectively as a result of G-CSF. It frequently helps neutrophil counts increase following chemotherapy or bone marrow transplantation.
Granulocyte and macrophage production and activity are stimulated by recombinant granulocyte-macrophage CSF. In some circumstances, such as those involving bone marrow transplantation, it has been utilized to strengthen immune responses and improve hematological recovery.[17]
These are but a few illustrations of recombinant cytokines and their uses. The therapeutic potential of recombinant cytokines in treating a variety of diseases, including cancer, autoimmune disorders, and infectious diseases, has been thoroughly investigated and developed.
CELLULAR THERAPY
Adoptive cell transfer (ACT)
ACT entails the injection of immune cells into a patient to boost their immune response against a specific disease, such as T-cells or NK cells. This can be accomplished by isolating and boosting a subset of the patient’s immune cells or cells from a donor.[14]
Dendritic cell (DC) vaccines
DCs are essential for triggering and controlling immune responses. DCs are removed from patients, loaded in the laboratory with certain antigens or tumor cells, and then reinfused back into the patient as part of a DC vaccination therapy. The re-infused DCs trigger an immune response against the intended antigens or tumor cells, resulting in an improved immunological response to the disease.
Therapy with NK cells
NK cells are a subtype of cytotoxic immune cells that may identify and eliminate cancerous or infected cells. NK cells from either the patient or a donor are isolated, expanded, and then infused into the patient during an NK cell therapy procedure. NK cells are able to directly target and eliminate tumor cells. Therapy with NK cells is a potential therapy of cancer.
CONCLUSION
This article provides a comprehensive review of immunotherapeutics, focusing on hybridoma technology. It explains how antibody-based treatments using hybridoma technology have revolutionized medicine by providing precise treatments for diseases such as cancer, autoimmune disorders, and infectious diseases. The process of hybridoma technology is described, involving the fusion of immortalized myeloma cells with B lymphocytes to create a population of cells that produce unique mAbs. Selected hybridoma cells are then grown in large numbers to isolate and produce mAbs for therapy. The article showcases successful applications of immunotherapeutics based on hybridoma technology in treating autoimmune conditions, viral infections, and cancer. It highlights the potential of mAbs to expand treatment options. It also discusses the differences between monoclonal and polyclonal antibodies and the various uses of hybridoma technology in research, diagnostics, therapy, vaccine development, and fundamental immunology investigations. Overall, the article emphasizes the significance of immunotherapeutics based on hybridoma technology in transforming the treatment landscape and enabling personalized and targeted therapies. Ongoing research and advancements in the field are continually expanding the potential applications of mAbs, offering innovative and effective treatments for a wide range of diseases.
Acknowledgment
The author would like to thank Professor Keval for his expert advice and encouragement throughout this article.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Immunotherapy In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK519046 [Last accessed on 2023 Mar 07]
- [Google Scholar]
- A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat Rev Immunol. 2020;20:651-68.
- [CrossRef] [PubMed] [Google Scholar]
- A review on cancer immunotherapy and applications of nanotechnology to chemoimmunotherapy of different cancers. Molecules. 2021;26:3382.
- [CrossRef] [PubMed] [Google Scholar]
- CAR T cell immunotherapy for human cancer. Science. 2015;359:1361-5.
- [CrossRef] [PubMed] [Google Scholar]
- Hybridoma technology; advancements, clinical significance, and future aspects. J Genet Eng Biotechnol. 2021;19:159.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of the progression of hybridoma technology: Methods, applications, advantages and drawbacks. Sci Technol 2020:2327-5790.
- [Google Scholar]
- Hybridoma technology: A brief review on its diagnostic and clinical significance Germany: ResearchGate; 2016.
- [Google Scholar]
- Therapeutic use of monoclonal antibodies: General aspects and challenges for drug delivery. Netherlands: Elsevier; 2017:807-33.
- [CrossRef] [Google Scholar]
- Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat Rev Cancer. 2015;15:457-72.
- [CrossRef] [PubMed] [Google Scholar]
- The future of immune checkpoint therapy. Science. 2015;348:56-61.
- [CrossRef] [PubMed] [Google Scholar]
- Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23-34.
- [CrossRef] [PubMed] [Google Scholar]
- Exploiting the curative potential of adoptive T cell therapy for cancer. Immunol Rev. 2014;257:56-71.
- [CrossRef] [PubMed] [Google Scholar]
- T cell homing therapy for reducing regulatory T cells and preserving effector T cell function in large solid tumors. Clin Cancer Res. 2018;24:2920-34.
- [CrossRef] [PubMed] [Google Scholar]
- Lippincott's illustrated reviews: Pharmacology (6th ed). View all formats and editions. Philadelphia, PA: Wolters Kluwer; 2015.
- [Google Scholar]
- Delivering type I interferon to dendritic cells empowers tumor eradication and immune combination treatments. Cancer Res. 2018;78:463-74.
- [CrossRef] [PubMed] [Google Scholar]






