Translate this page into:
The potential seaweed resources assessment: Its cultivation prospect and future biofuel feedstock

-
Received: ,
Accepted: ,
How to cite this article: Olanrewaju OS, Shukor H, Guerrier G, Bagchi D, Aruoma O, Ismail SK. The potential seaweed resources assessment: Its cultivation prospect and future biofuel feedstock. Am J Biopharm Pharm Sci. 2024;4:3. doi: 10.25259/AJBPS_15_2023
Abstract
Global issues on energy and fuel for sustainable development industrial and household system are a major concern in the world economy. Biofuel is a renewable energy source that can potentially be a replacement for fossil fuels. The utilization of algal biomass or seaweed organic matter is a good source of ethanol, methanol, biobutanol, and biodiesel. Malaysia’s waters recorded approximately 400 species of macroalgae, with several species found to be a potential source for biofuel application. An expedition study for the seaweed natural resources was carried out in December 2016 in coastal areas in Johor and Melaka, Peninsular Malaysia. Green seaweed, Ulva, large brown seaweed, Sargassum, and Gracilaria (red seaweed) are found in the selected study areas and these genera are listed as biofuel resources in the literature. The design of offshore mariculture system specifically for seaweed farming for biomass production for biofuel. The prototype was constructed and deployed in Bidong Island, Terengganu in 2016 before the monsoon. At the end of the monsoon period, the prototype is still stable in situ. Selected seaweed species with biofuel potential were trial cultivated on the system and the growth performance of the particular species was monitored. The suggested species for biofuel are Gracilaria and Ulva, while Kappaphycus is targeted for the food industry. Gracilaria and Ulva were also studied for their bioremediation potential and suitability to grow on the system. Gracilaria spp. was able to grow on the system, indicating the designed system is feasible for biomass production of seaweed. Further, the selected seaweed species can function as biofilters for the nutrients in the environment and acceptability for a wide salinity range meaning that the system can be applied in different locations such as estuary, inshore, or offshore. Seaweed biofuel and its subsequent advantage related to pollution-free energy generation is of critical importance.
Keywords
Biofuel
Seaweed
Cultivation
Open water farming system
Species
Macroalgae
Seaweed resource
INTRODUCTION
Seaweed macroalgae are marine macroalgae that are divided into three different phyla which are phylum Chlorophyta (green seaweed), Ochrophyta (brown seaweed), and Rhodophyta (red seaweed).[1-4] Seaweeds have broad variety, fast growth, good reproduction in low land and water requirement, with their high oil content, are promising source of renewable energy.[4,5] Almost all of the world’s biomass comes from a relatively small number of species in the orders Laminariales and Fucales. The total wholesale value of dried brown seaweed collected from the wild or cultivated worldwide is <100 million USD. The main red algal biomass in the world is provided by Corallinaceae and Gigartinaceae,[6] while the number of species of AlgaeBase shows that there are about 4,500 species of chlorophyll, including about 550 species of Trebouxiophyceae (mainly living in air and freshwater), and 2,500 species of Chlorophyceae (mainly living in freshwater), 800 species of Bryopsidophyceae (algae), 50 species of Dasycladophyceae (algae), 400 Siphoncladophyceae (algae), and 250 marines Ulvophyceae (algae). Chlorophyll is entirely freshwater and includes 3,500 species. In addition, the number of species of AlgaeBase shows that there are about 10,000 species of algae, including about 6,500 species of Rhodophyta and red algae.
Due to these properties, it can be a suitable renewable and sustainable source for energy production such as biofuel. According to Tabarsa et al., seaweed is a renewable source of the ocean with potential food applications and also is an alternative source of polyunsaturated fatty acids such as omega-3.[7] Besides, the fatty acid in seaweed lacks a pleasant odor and has the potential for oil refining.[8] Fuel is an important energy source for human mankind that is mainly used in transportation, industry and other daily societal activities. A high level of carbon dioxide leads to global warming and the greenhouse effect. Moreover, petroleum resources are declining, so another relevant source is critically needed.[8] Biofuel is an excellent substitute for fossil-fuel-derived energy sources, as it can be produced by abundant supplies of renewable biomass such as vegetable oils and algae.[9] Seaweed microalgae are vegetable matter that can be attained from photosynthesis. This bio-feedstock can produce biomass that is mainly composed of lipids, carbohydrates, and proteins using sunlight and CO2[10] which is renewable, sustainable, biodegradable, carbon-neutral for the whole life cycle, and environmentally friendly.[8] The organic molecules, carbohydrates, and lipids contents in seaweeds can be used for conversion to various fuels and chemicals[11] such as biodiesel, bioethanol, biomethanol, and biohydrogen.[8]
In Malaysia, Kappaphycus and Eucheuma are the only red seaweeds commercially produced and restricted in Sabah waters.[12] Trial cultivation on both species was done on the east coast and west coast of Peninsular Malaysia. However, successful farming of these species in Peninsular Malaysia is not documented. Various culture methods have been implemented to reproduce the algae either through vegetative propagation or spore seedling. In Malaysia, experimental cultivation of Gracilaria has been conducted in the Middle Bank, Penang and Ban Merbok, Kedah through line – spore-settling, pond, and raft culture methods.[13] Cultivation Trials of selected Gracilaria species have been conducted at the University of Malaysia Terengganu Hatchery as part of industrial research development to commercializing Gracilaria cultivation involving large-scale farming that will meet Malaysia National Key Economic Area (NKEA) Agenda. This paper documents several species of seaweed from the natural population obtained through a scientific expedition in 2016 on the Melaka and Johor coasts of the Peninsular. Given that limited investigations have been reported for biodiesel production from marine macroalgae or seaweed, the objectives of the study presented were to screen collected species as potential biofuel sources and mass production of these species using offshore farming practices. Besides the species verification, the paper also discusses the use of seaweed for biofuel and the state of the art on the possibility of replacing algae with fossil fuel.
SEAWEED AND ITS PROCESS CONVERSION TO BIOFUEL
Seaweed is an autotrophic, marine organism that produces energy like most other plants by way of photosynthesis.[9] Macroalgae or “seaweeds” are multicellular plants that are usually grown in salt or freshwater. They are often fast-growing and can reach sizes of up to 60 m in length. Seaweed or macroalgae are more resistant to predators and environmental conditions than microalgae. They are classified into three broad groups based on their pigmentation: (i) brown seaweed (Phaeophyceae); (ii) red seaweed (Rhodophyceae); and (iii) green seaweed (Chlorophyceae). Seaweed is mainly utilized to produce food and the extraction of hydrocolloids.[14] Seaweeds or macroalgae belong to the lower plants, meaning that they do not have roots, stems, and leaves. Instead, they are composed of a thallus (leaf-like) and sometimes a stem and afoot. Some species have gas-filled structures to provide buoyancy. In their natural environment, they will grow on rocky substrates and form stable, multilayered, and perennial vegetation capturing almost all available photos. Because seaweed is fixed to its substrate, values for maximum productivity maybe 10 times higher for a seaweed stand than for a plankton population and can be as high as 1.8 kg/cm2/y. The maximum chlorophyll content is 3 g/m2 on an illuminated surface, corresponding to an algal biomass of about 10 kg m−2. The productivity of plankton is much lower because most of the photons are absorbed or scattered by abiotic particles, and the algae are so thinly distributed.[14] Approximately 200 species of seaweeds are used worldwide, about ten of which are intensively cultivated, such as the brown algae Laminaria japonica and Undaria pinnatifida, the red algae Porphyra, Eucheuma, Kappaphycus, and Gracilaria, and the green algae Monostroma and Enteromorpha.[14,15]
It is reported that several fuels have been produced from algae-based biomass of seaweeds such as biohydrogen, biodiesel, jet fuel, and bioethanol.[16,17] These biofuels were produced using carbohydrates and lipids content in seaweed biomass which was produced by sunlight, CO2, and certain micronutrients, especially nitrogen (N) from the medium (photoautotrophic). The source of carbon in the form of CO2 and light are the key factors that influence the maximum growth of seaweed [Figure 1]. In addition, the small composition of hemicellulose and negligible lignin in the seaweed makes it biofuel-based algae that can exclude the pretreatment process, and this will enhance its hydrolysis and fermentation process efficiency.[18] Other than photoautotrophic seaweed, other types of seaweed can be found such as heterotrophic and mixotrophic. These types of seaweed are different in terms of their cultivation method. Heterotrophic seaweeds use the organic compound as their carbon and energy sources without external light source requirements.[17] While a combination of photoautotrophic and heterotrophic which is called mixotrophic applied to both inorganic and organic components as well as can overcome the light limitation.
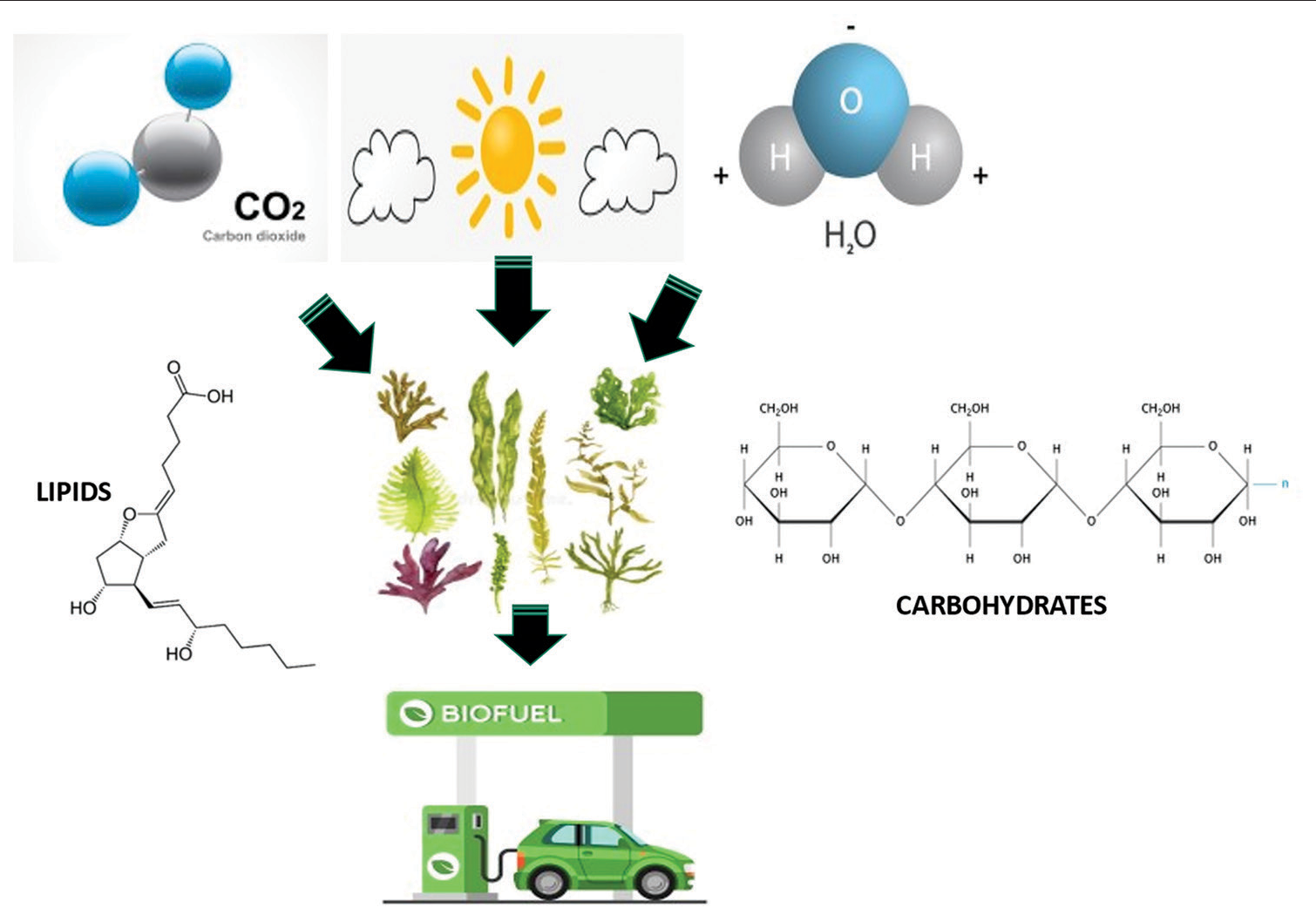
- Lipids and carbohydrate source from seaweed for biofuel production. The source of carbon in the form of CO2, and light are the key factors that influence the maximum growth of seaweed.
Seaweed to biofuel process conversion can be divided into two main pathways. The first one is the lipid or oil-based biofuel pathway, which generally focuses on biodiesel production and the second pathway is the carbohydrate-based seaweed pathway. This second pathway can bring us to several types of biofuel productions such as bioethanol, bioethanol, and biogas (biohydrogen and biomethane) production. The conversion technologies for utilizing seaweed biomass are thermochemical and biochemical conversion. Thermochemical conversion includes pyrolysis and gasification process, while biochemical reaction includes anaerobic digestion and transesterification.[19]
Lipid or oil-based seaweed for biodiesel production
Kalscheuer et al., reported that biodiesel is biodegradable, with less CO2 and NOx emissions. It is produced from the transesterification process where the triacylglycerols will be converted into monoalkyl esters of long-chain fatty acids with short-chain alcohols such as fatty acid methyl esters (FAMEs) (biodiesel).[20] Transesterification is a chemical process that involves reversible reactions between triglycerides from vegetable oil and alcohol with the presence of a catalyst such as acid and alkali or biological catalysts with an alcohol to produce esters and by-products.[21] Examples of alcohols that can be used include methanol, ethanol, propanol, and butanol, but the most used is methanol. Methanol is cheap and has a favorable physical and chemical nature (shortest-chain alcohol). In addition, transesterification is a simple and cost-effective process that produces biodiesel with low viscosity. It also produces glycerol that has commercial value as a by-product.[21] For instance, the production and consumption of biodiesel resulted in a 78.5% reduction in carbon dioxide emissions.[19]
Seaweeds are a potential alternative and renewable source for conventional feedstocks for biodiesel production.[22] Lipid or oil-based seaweeds are suitable for esterification/transesterification reaction of biodiesel production which could meet the global demand for transport fuels. Biodiesel can be produced from seaweed due to its lipid content [Figure 2]. Fish oil for nutraceutical use has been the major source of docosahexaenoic acid (DHA), it might be produced using marine micro- and macro-algae species. Marine microorganism species might contain large quantities of DHA and be considered a potential source of this important fatty acid. Properly harvested oil can be converted into biofuels.[23]
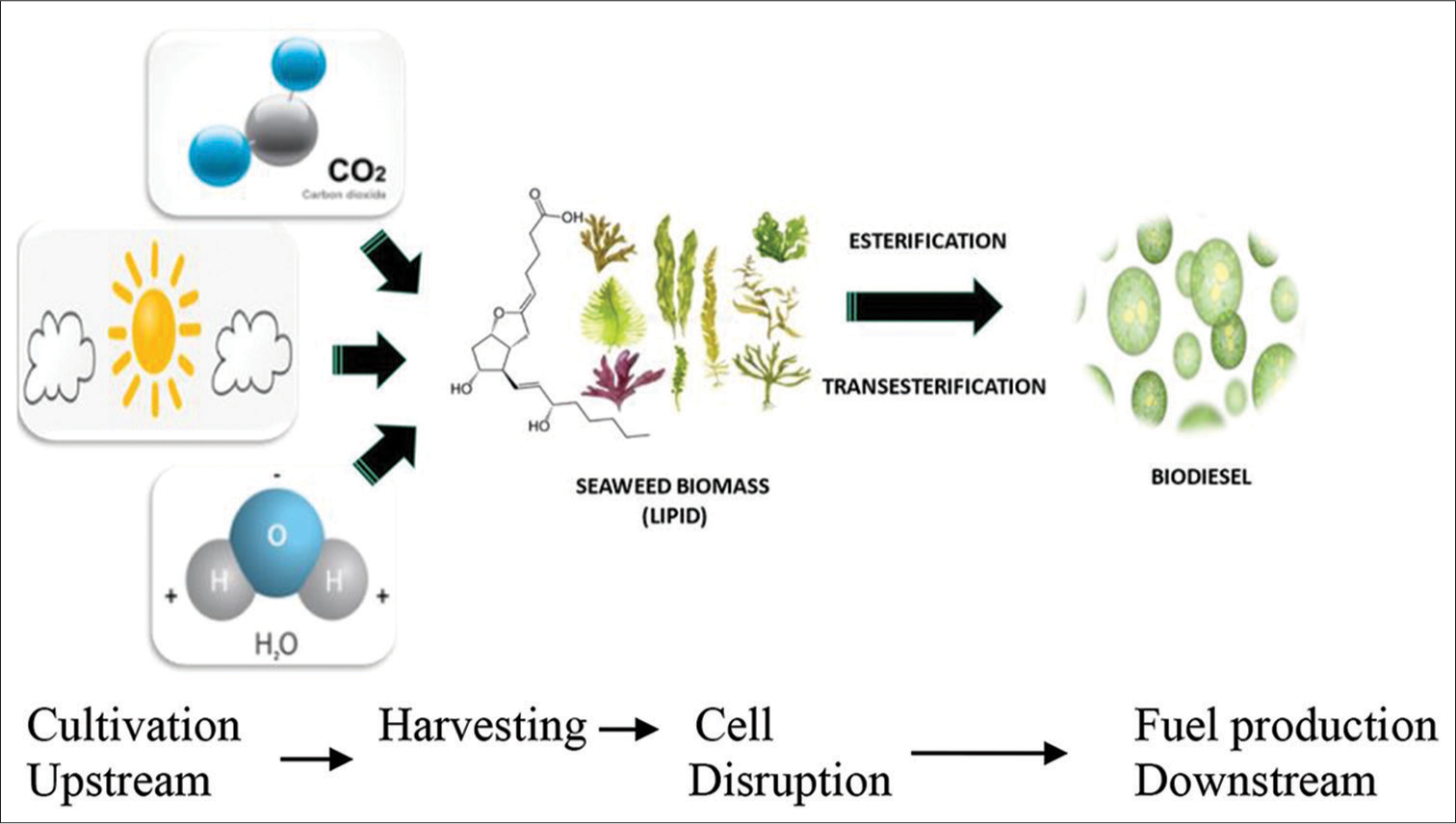
- Seaweed for biodiesel production using the lipid content in its biomass.
Algae were first explored as a fuel alternative in 1978 under President Jimmy Carter. Gas prices skyrocketed, lines at the pump were endless, and the government was looking to help ease the crisis. The increasing energy demand is expected to provide the much-needed financial incentive to work on improving production and redoubling the efforts to use renewable sources for energy effectively. The incremental barrel of hydrocarbon is getting heavier, and many also believe that the incremental conventional cubic foot of natural gas is getting sourer. This will place an ever-increasing burden on processing equipment. This represents an excellent opportunity to virtually transform the current paradigm of production and refining. This game-changing technology can be applied to deriving usable end products from a biodegradable source of energy. The successful application of the technology could blur the distinction between upstream and downstream operations.[24] Algae-based feedstock (seaweeds) can be grown on non-arable land (without competition with food crops) using only sunlight and water for growth. This category of aquatic organism has long been considered to have the potential for producing transportation fuels, particularly biodiesel, which is produced by the chemical transesterification of fatty acids.
Algae or seaweed are also the fastest growing of all plant species on earth. These two characteristics give seaweed an exceptional biomass output and fuel conversion potential compared with land crops in per unit area comparisons. Properly supported, large-scale seaweed cultivation presents an achievable opportunity to significantly replace fossil fuel usage. According to Aitken et al., a life cycle assessment study has been initiated for the evaluation of the potential of macroalgae for fuel production.[25] This study reveals that macroalgae need nutrients (N, P, microelements) associated with nutrient and cultivation energy. Variance analysis has shown that if the energy of nutrients is taken into consideration, the energetic balance may be negative. Therefore, to avoid such a huge amount of energy input, either effluent water from aquaculture plants should be used, or some selected municipal waters.
Seaweed works as a purifying agent and treated water can be either re-circulated to the fishpond or immitted into natural basins without paying any penalties. Such use of effluent water, by the way, generates a credit to the process that may be ultimately considered. The focus on the use of biomass as an alternative feedstock to fossil fuels is intensifying due to its role in reducing CO2 emissions. At present, many technologies are under investigation for the utilization of biomass both for power generation and to produce biooil for transportation and chemical commodities. Two of the main issues of biomass utilization are the security of supply and the development of an infrastructure capable of maintaining the supply of biomass of sufficient quality.[26] The upstream and downstream sides of the energy sector, like most other industries, face a challenge in the areas of exploration, production, and refining. These also pose unique opportunities to re-evaluate the processes and develop game-changing technologies to improve overall efficiency. The game-changing technologies should improve the economics of the process and help to improve the safety and reliability of the activity. For example, if hazardous materials, such as CO2 and H2S, are treated downhole and not brought to the surface; then, this not only improves the health and safety but also reduces the operating cost by eliminating the need to treat and dispose of these materials. The process should be optimized so that the incremental savings are greater than the incremental cost.[24,27]
Production of biodiesel from seaweeds generally involves several steps [Figure 2] which make it less attractive when starting from cell drying to disruptions of cells, lipid or oil extraction, transesterification process, and purification of biodiesel produced.[28] Nevertheless, the current technology of in situ (lipid extraction and transesterification in a single step) transesterification process makes this biodiesel production more feasible on an industrial scale.[29] The key raw materials for biodiesel production are oils or lipids. Tripathi et al., reported that the fatty acid composition of seaweed (stearic acid, palmitic acid, and oleic acid) is like the biodiesel standard.[30] Normally, the yield of lipids produced from this algae-based biomass, especially microalgae is in the range of 2.4–62% of dry biomass, [Table 1].[30-33] Methyl ester (biodiesel) produced after the transesterification process is high in unsaturated fatty acids, which is one of the prerequisites for being used as biofuel.[30] Mata et al., in 2010, stated that, from 1 kg of oil extracted from algae-based biomass, 1 kg of biodiesel can be produced.[32]
Carbohydrate-based seaweed for sugar-based biofuels production
Sustainable biofuel alternatives have been deeply investigated by many researchers to find a potential resource to replace fossil fuels. The potential of algae-based biomass as a third-generation feedstock for biofuel production continues to be investigated. Green algae contain large amounts of sugars in the form of complex carbohydrates. Agwa et al., reported that Chlorella vulgaris consists of approximately, 37–55% of cell dry weight of carbohydrates.[34]
This high content of carbohydrates shows that algae-based biomass can be one of the potential resources to produce other types of biofuels, especially sugar-based biofuels such as bioethanol and biobutanol. However, this algae-based biomass or seaweed needs to go through pretreatment and hydrolysis to turn this complex carbohydrate into a simple form of sugars for bioethanol or biobutanol production [Figure 3]. The study by Yanagisawa et al., reveals that seaweeds of Ulva pertusa, Alaria crassifolia, and Gelidium elegans have no lignin composition of polysaccharide.[35] This shows that seaweed did not require any pretreatment process before their hydrolysis. Several hydrolysis methods have been successfully applied for bioethanol production using seaweed as the sole carbon source. Table 2 shows the yield and different types of seaweed or macroalgae used for bioethanol production using several types of hydrolysis methods.[5,36-40] Fermentation of seaweed biomass will be based on polysaccharide composition since the composition of seaweed will vary from species to species even though the polysaccharide in the cell walls of seaweeds is generally composed of cellulose and hemicellulose.
Seaweed biomass can be considered a new promising feedstock to produce biofuels, especially bioethanol production [Figure 3]. Many of the third-generation biofuels can be grown on non-arable land that are subject to harsh conditions and different environments, such as using unused water space and wastewater as the main medium for the source of nutrients. Their fastest growth compared to any oilseed plants can reach more than 59% lipid per dry weight for certain types of species for biodiesel production and their biomass with high polysaccharide content can be used to produce a wide variety of bioproducts especially other types of biofuels such as bioethanol and biobutanol which can promise us for higher net energy returned (NER) compare to other biofuels and viable feedstock for large-scale production of biofuels without competing with land-based food crops. Thus, there has been a great deal of development of systematic algae-based biofuels production systems for the sustainability of future renewable biofuels.

- Seaweed for bioethanol and biobutanol production.
| Seaweeds name | Pretreatment and hydrolysis method | Yield (%) | References |
|---|---|---|---|
| Laminaria japonica | No Pretreatment+Enzymatic hydrolysis (endoglucanase+β-glucanase+amyloglucosidase) | 19.6 | [36] |
| Gracilaria verrucosa | No Pretreatment+Enzymatic hydrolysis (cellulase+β-glucosidase) | 43 | [37] |
| Gracilaria salicornia | 2% H2SO4+Enzymatic hydrolysis (Cellulase) | 7.9 | [5] |
| Laminaria digitata and Saccharina latissima | Shredding and saccharification | 13.2 and 0.47 | [38] |
| Saccharina japonica | H2SO4+Enzymatic Hydrolysis (cellulase+β-glucosidase) | 11.10 | [39] |
| Laminaria japonica | 0.1 M H2SO4+Enzymatic Hydrolysis (cellulase+cellobiose) | 11.3 | [40] |
State of the art of cultivation of seaweed biofuel species
Continuous use of petroleum-sourced fuels is now widely recognized as unsustainable due to depleting supplies and the contribution of these fuels to the accumulation of carbon dioxide in the environment. Renewable, carbon-neutral, and transport fuels are necessary for environmental and economic sustainability. Algae have emerged as one of the most promising sources for biodiesel production. It can be inferred that alga grown in CO2-enriched air can be converted to oily substances. Such an approach can contribute to solving major problems of air pollution resulting from CO2 evolution and future crisis due to a shortage of energy sources. The government’s use of upstream R&D investments and downstream incentives for renewable energy is intended to achieve commercial breakthroughs in biofuels, batteries, fuel cells, hydrogen, solar, and wind energy. The private sector has reacted to these policy instruments with a significant increase in renewable energy R&D and commercial investment. As the private sector’s exposure to renewable energy markets increases, the public sector will increasingly be pulled by special interests in the direction of insuring against the downside risks of clean energy investments.[41,42]
Tyler and McGlathery reported that Macroalgae or seaweed, often the dominant primary producers in shallow estuaries, can be important regulators of N cycling.[43] Like phytoplankton, actively growing macroalgae release N to the water column, and the quantity or nature of this release remains unknown. Using 15N labeling in laboratory and field experiments, we estimated the quantity of N released relative to assimilation and gross uptake by Gracilaria vermiculophylla (Ohmi) Papenfuss (Rhodophyta, Gracilariales), non-native macroalgae. Field experiments were carried out in Hog Island Bay, a shallow back-barrier lagoon on the Virginia coast where G. vermiculophylla makes up 85–90% of the biomass. There was good agreement between laboratory and field measurements of N uptake and release. Daily N assimilation in field experiments was correlated with seasonal and local N availability. The average rate of N release across all sites and dates was 67% of gross daily uptake and varied among sites and seasons (range 33–99%). The release was highest when growth rates and nutrient availability were low, possibly due to senescence during these periods. During summer biomass peaks, the estimated N release from macroalgae mats was as high.
The design and model testing of offshore aquaculture floating structures for seaweed oceanic plantations has been reported by Sulaiman Olanrewaju et al.[44] Indeed, seaweed farming has become one of the economically important natural resources. The existing cultivation system for seaweed is not suitable for deployment in most deep and open-water areas. Moreover, the current cultivation system is not environmentally sustainable and economically unstable. The design of the offshore floating structures is scientifically based on the improvement of the Long Line System for commercializing scale seaweed farming. Some key factors in the design, prototype, and testing of floating offshore structures considered in the development of ocean farming technology systems are discussed. Sulaiman Olanrewaju reported the mooring analysis for a very large offshore aquaculture ocean plantation floating structure.[45] Aquaculture activities are inherently undertaken in proximity to the coastline and near the shore. Mounting constraints and environmental impact concerns necessitate the development of offshore aquaculture. Challenges include achieving a reliable structural integrity and mooring system design for ultimate state limit, fatigue state limit, and accidental and progressive state limit against environmental loading and accidental loading. To avoid mooring system failure, selecting an appropriate breaking strength and limit state for mooring system components is essential. The system design accounts for forces and environmental loadings. An evaluation of optimum mooring performance in a wave, wind, and current loadings on mooring components anchor, buoy, and riser elements that are involved in the dynamic system sought to establish suitable safety factors for large offshore aquaculture floating structures and address the risks associated with seaweeds, biofuel renewable and alternative technologies.[46-48]
SCIENTIFIC EXPEDITION FOR COLLECTION OF LOCAL SEAWEED SPECIES IN PENINSULAR MALAYSIA AND CULTIVATION TRIAL AT BIDONG ISLAND, TERENGGANU
Collection of seaweed in natural stock
Bidong Island is an island in Kuala Nerus District, Terengganu, Malaysia in the South China Sea. A scientific expedition was carried out in December 2016 mainly focused on coastal areas of Melaka in Malacca Strait and Johor in Tebrau Strait. The locations of sampling are shown in Figure 4. Five selected localities were surveyed for their natural seaweed populations. Sampling was done during the low tide period. Samples were collected by hand. Further, cleaning, sorting, and storage in cold conditions before being transferred back to the laboratory for analysis. Fresh and healthy plants were kept alive in clean seawater as seaweed stock for cultivation purposes.[49]
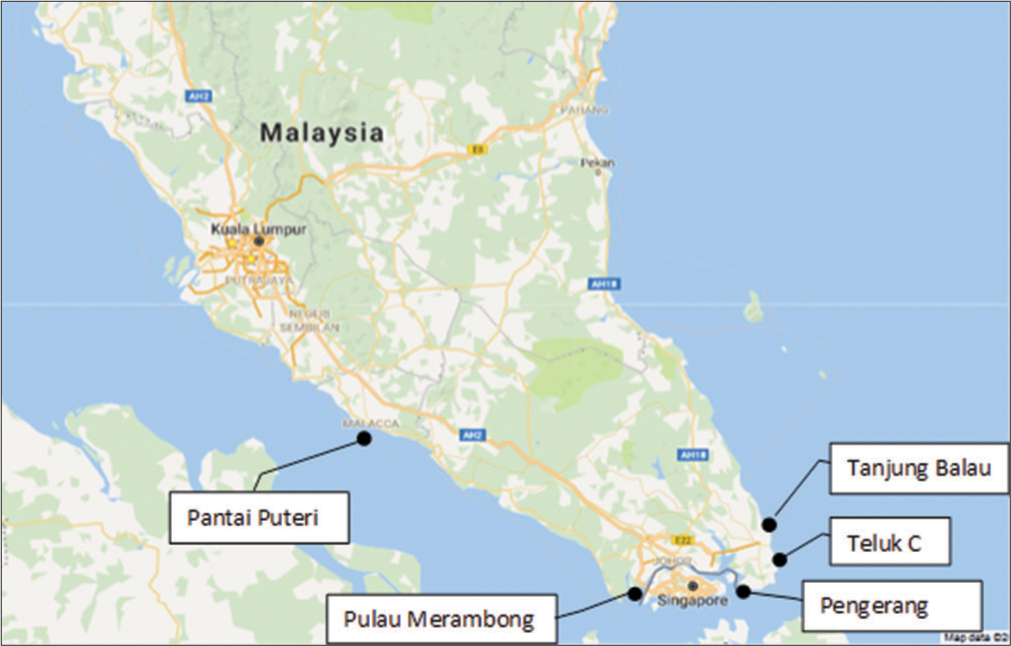
- Sampling localities of seaweed during a scientific expedition in 2016.
Species identification
Complete plants were selected and preserved as dried herbarium specimens. Identification was done according to available taxonomic references. Later, these specimens were cataloged and kept in marine algal reference collection in the Herbaria of University Malaysia Terengganu, Central Laboratory as voucher materials.
Seaweed trial cultivation
Freshly collected samples were acclimatized for 6 weeks and healthy thalli were chosen for trial cultivation. The cultivation was focused on Ulva and Gracilaria species using a designed offshore farming system deployed in Bidong Island, Terengganu.[50]
Species of Ulva and 2 Gracilaria were measured for their fresh weight (as initial weight) and kept in a planting basket with dimensions (26 [L] × 19 [W] × 9 [H] cm) and volume (4.45 L) and covered with fishing net (1.5 cm mesh size). Stocking density for Ulva is 2.5 g/L and 5.0 g/L for both Gracilaria species and triplicates of samples were studied. Three baskets were tied on the same line and parallel with another two lines also with three baskets each. After 2 weeks, measurement of growth was conducted biweekly in situ.
The specific growth rate (SGR) for seaweed was determined as the following formula:
SGR (%/day) = ([LN W2–LN W1]/T2–T1) * 100% (1)
Where, W2 = final weight, W1 = initial weight, T2 = final time, T1 = initial time
The doubling time for seaweed biomass increased 2 times was calculated as the followed formula:
Doubling time (day) = 50/SGR
Hydrological parameters
Salinity was checked using a handheld refractometer and dissolved inorganic nutrients (ammonium, nitrite, nitrate, and phosphate) were analyzed following the standard chemical methods for seawater analysis.[45]
Biofuel seaweed species in Malaysia
The survey was done in December 2016 in five selected coastal areas in Melaka and Johor. The green seaweed Ulva sp. was found in two localities in Johor and can be a source of bioethanol and biobutanol. Gracilaria sp. (red seaweed) with the potential for bioethanol is found abundantly in Melaka and Johor.[25] Giant tropical brown seaweed, Sargassum sp. was a perennial species found in Melaka and Johor with potential for bioethanol, biomethane, and bio-oil production.[51] Table 3 summarized the seaweed species with biofuel potential found in different localities of Peninsular Malaysia.
| Species | Pantai puteri | Pulau merambong | Pengerang | Teluk C | Tanjung balau |
|---|---|---|---|---|---|
| Ulva sp. | √ | √ | |||
| Gracilaria sp. | √ | √ | √ | √ | |
| Sargassum sp. | √ | √ | √ | √ |
Figure 5 shows the species pictures collected during the expedition as potential biofuel species. Ulva sp. known as sea lettuce is an edible green alga in the family of Ulvaceae. It is a thin flat green alga that can have a length of 18–30 cm. While Gracilaria sp. is a red type of seaweed that is commonly found in warm waters and traditionally cultivated as a source of agar. Sargassum sp., in contrast, is a brown type of seaweed and generally appears brown or dark green. It will spend its life on the ocean’s surface and float in large masses. This finding reveals that Gracilaria sp. and Sargassum sp. are the common types of seaweed growth in Malaysia’s oceans.

- (a) Ulva species from Pulau Merambong; (b) Ulva reticulata from Pengerang; (c) Gracilaria sp.1 from Melaka; (d) Gracilaria sp.2 from Pulau Merambong and Teluk C, Johor; (e) Gracilaria sp.3 from Pengerang, Johor; (f) Sargassum sp. from Melaka; (g) Sargassum sp. from Pulau Merambong; (h) Sargassum sp. from Tanjung Balau.; and (i) Sargassum sp. from Teluk C.
Species identification
The flat sheet of Ulva collected in Pulau Merambong and Pengerang was two different species. The samples from Pulau Merambong have reported a new record as Ulva species. The samples from Pengerang were identified as Ulva reticulata and matched with taxonomic references. Three Gracilaria species were identified as Gracilaria sp. 1 from Pantai Puteri, Melaka, Gracilaria sp. 2 from Pulau Merambong and Teluk C, and Gracilaria sp. 3 from Pengerang. The large Sargassum was identified as four different species in Pantai Puteri, Melaka, Pulau Merambong, Teluk C, and Tanjung Balau, Johor. This species was further grown in the offshore farming system to determine its SGR.
Growth of seaweed in offshore farming system
Table 4 shows the SGR of cultivated species for 14 and 28 days with different stocking densities. The growth rate for Ulva was not available since all samples were lost in the planting baskets. A possible reason is samples of Ulva were not adapted to a new environment. The growth rate for both Gracilaria sp.1 and Gracilaria sp.2 with 3.84%/day and 4.97%/day, respectively [Figures 6 and 7]. Gracilaria spp. was archived doubling time in 10–13 days showing the environment very suitable for its growth. Abundant new branching tips were observed on seaweed thalli and branches became thicker.
| Species | Stocking density (g/L) | SGR (%. Day-1) | Doubling time (day) | |||||
|---|---|---|---|---|---|---|---|---|
| Day 14 | Day 28 | Average | ||||||
| Gsp. 1 | 5.0 | 3.84 | ±1.34 | 2.15 | ±0.62 | 3.00 | ±0.97 | 17 |
| Gsp. 2 | 5.0 | 4.97 | ±0.15 | - | - | 10 | ||
| Ulva | 2.5 | N/A | N/A | N/A | N/A | |||
| Gsp. 2 | 7.5 | 3.31 | ±1.35 | - | - | 15 | ||
| Gsp. 2 | 2.5 | 3.42 | ±0.82 | - | - | 15 | ||
N/A: Not available, Gsp. 1: Gracilaria sp. 1, Gsp. 2: Gracilaria sp. 2, SGR: Specific growth rate

-
Gracilaria sp.1.

-
Gracilaria sp.2.
All Gracilaria samples were covered with an epiphytic organism such as other macroalgae and invertebrates. Besides, the net covering and planting basket are also attached with tiny brownish fouling invertebrates or microalgae. After planting baskets were cleaned with seawater, the seaweed samples were placed back in the same planting units. Since three baskets for Ulva were empty, Gracilaria sp.2 with a stocking density of 2.5 g/L was replaced in each basket [Figures 6 and 7]. The planting of seaweed baskets is now largely done using twist ties instead of the main culture lines raffia.
The growth rate for Gracilaria sp.1 on day 28 decreased from 3.84%/day to 2.15%/day. Gracilaria sp.2 for two stocking densities 7.5 g/L and 2.5 g/L showed different growth rates which were 3.31%/day and 3.42%/day, respectively, on day 14. Gracilaria spp. was archived doubling time in 15–17 days showing the environment still suitable for its growth. New branching tips were observed to be bigger on seaweed thalli, and branches became thicker as well. However, the growth rates for both Gracilaria spp. decreased from day 14 to day 28 with possible reasons: nutrients, light, and space competition between seaweed and fouling organisms; seaweed stock used for cultivation was kept in the hatchery for two months and getting older before cultivation started; and tropical temperature making growth synergy slow due to high metabolism rate.
All Gracilaria samples were covered with epiphytic organisms such as micro-and macro-algae [Figures 8 and 9] and invertebrates [Figure 10]. Besides, the net covering and planting basket was also attached with tiny brownish fouling invertebrates or microalgae and became abundant on day 28 for all baskets. Since the fouling organisms were too abundant and strongly attached to the net covering, after fresh weight measurement all baskets without cleaning planting baskets and tied back to the prototype for following growth observation.[49,50]

-
Gracilaria sp.2 was replaced by an empty basket used for Ulva.

- Fouling and epiphytic macroalgae.

- Some invertebrates and vertebrates.
Fouling and epiphytic organisms were found such as invertebrates: Share, copepod, roundworm, shrimp; vertebrates: fish (not considered as fouling); microalgae: diatom mainly pennate diatom and macroalgae: Unidentified cyanobacteria; Bryopsis, Chaetomorpha, and Cladophoropsis (green seaweed); and Acanthophora, Herposiphonia, Laurencia, Polysiphonia, and Tolypiocladia (red seaweed).[49,50]
The offshore farming system was designed, and simulation tests were done. After simulation tests, the system was planned for deployment in front of Pantai Pasir Cina, Pulau Bidong, and Terengganu. The dimension of the system is 2.0 m × 2.0 m with four large buoys at each corner, two small floats for each marginal line, and three small floats on three parallel lines for seaweed planting. The length of each mooring was 5.4 m, and the depth was 1.4 m during low tide.[49,50]
The deployment of the system was before the Northeast monsoon season in October 2016. After the monsoon, the system remained in its original position, which was considered feasible. Biofuel species such as Ulva and Gracilaria were trial cultivated on the system. However, Ulva was not able to adapt to this new environment. Both Gracilaria species performed a good growth rate and fast doubling time for biomass production as well compared to some commercial farming practices. Inorganic ammonium, nitrate, and phosphate were low in concentration except nitrate was much higher than the safety limit suggested by DOE, Malaysia [Figure 11]. A broad range of salinity 32–35% [Figure 12] was tolerant by seaweed with growth. In the future, the food species Kappaphycus will be cultivated to understand its growth performance in this system.

- Dissolved inorganic nutrient availability in Bidong waters during pre-monsoon and post-monsoon.
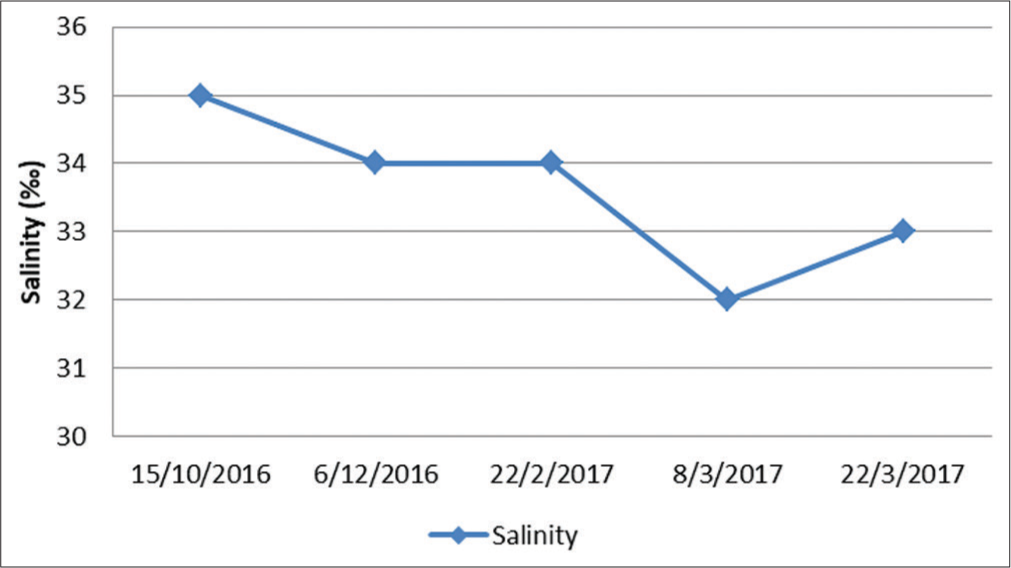
- Salinity range of Bidong waters during the cultivation period.
Plant oils or triglycerides are converted through the transesterification reaction with methanol and base catalyst to produce FAME or biodiesel. Production of biodiesel from plant oil is a renewable, sustainable, and alternative to petroleum-based fuel. Algae oil (AO) from macroalgae has the potential to become a sustainable fuel source as biodiesel. The lipid contents or oil in algae, once extracted and purified, represent an excellent sustainable feedstock for biodiesel production. The Ulva species from Sabah was reported as a potential biodiesel source.[52] The AO was extracted by the chemical extraction method. The transesterification reaction of AO with methanol and base catalyst was used to produce biodiesel. The engine performance test showed a slight increase in specific fuel consumption, but biodiesel blends showed higher brake power. The emission of CO, HC, and NOx reduced as the biodiesel blend percentage increased over the engine speed range.
Seeweed farming and aquaculture development require sustainable surveillance, the review of Xing et al.[53] is seminal. The multi-sensor remote sensing technology can be a part of the surveillance of coastal ocean for hazard management and engage sustainable farming strategies involving cultivation infrastructure systems to support high productivity, and source of seaweed biofuels supply chain.[53,54]
CONCLUSION
Seaweed cultivation can serve the conservation and commercialization purposes for biofuel and fossil fuel development. Improper management of the discovery and availability of useful resources can inadvertently lead to overexploitation and over-harvesting. In such cases, cultivation must play an important role in maintaining the resources in a sustainable yield and preventing extinction. Another function of cultivation is to reproduce a resource in a mass quantity and commercialize it to reap profit. Cultivation of potential biofuel seaweed species, Gracilaria in Malaysia as proposed in this work will be in accord with these two purposes. Seaweed can provide a solution to maximize energy production and zero pollution in our society. Research on species identification, optimization of the use of seaweed in this direction, and the performance of auto engines are key to delivering the energy required. Seaweed is nature’s gift to the environment. It has the potential to answer energy crisis questions and engage the notion of total replacement of fossil fuel, which will lead to the next generation of energy production, supply, and adequate pollution control. This is potentially the best way of obtaining optimized production, high performance and zero pollution in the environment.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
Dr. Okezie I. Aruoma and Dr. Debasis Bagchi are on the editorial board of the Journal.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Seaweed production: Overview of the global state of exploitation, farming and emerging research activity. Eur J Phycol. 2017;52:391-406.
- [CrossRef] [Google Scholar]
- Risk analysis of aquaculture farming system: Towards reliable sea farming Germany: Lambert Academy Publishing; 2016.
- [Google Scholar]
- Development of bioenergy technologies: A scientometric analysis. Heliyon. 2023;9:e20000.
- [CrossRef] [PubMed] [Google Scholar]
- Two-stage hydrolysis of invasive algal feedstock for ethanol fermentation. J Integr Plant Biol. 2011;53:246-52.
- [CrossRef] [PubMed] [Google Scholar]
- AlgaeBase In: World-wide electronic publication. Galway: National University of Ireland; 2014.
- [Google Scholar]
- Fatty acids, amino acids, mineral contents, and proximatecomposition of some brown seaweeds. J Phycol. 2012;48:285-92.
- [CrossRef] [PubMed] [Google Scholar]
- Valuable products from biotechnology of microalgae. Appl Microbiol Biotechnol. 2004;65:635-48.
- [CrossRef] [PubMed] [Google Scholar]
- Macroalgae and microalgae as a potential source for commercial applications along with biofuels production: A biorefinery approach. Renew Sustain Energy Rev. 2016;55:909-41.
- [CrossRef] [Google Scholar]
- Impact of cultivation condition and media content on Chlorella vulgaris composition. Adv Pharm Bull. 2019;9:182-94.
- [CrossRef] [PubMed] [Google Scholar]
- Marine macroalgae: An untapped resource for producing fuels and chemicals. Trends Biotechnol. 2013;31:70-7.
- [CrossRef] [PubMed] [Google Scholar]
- Commercial varieties of Kappaphycus and Eucheuma in Malaysia. Malays J Sci. 2010;29:214-24.
- [CrossRef] [Google Scholar]
- Preliminary results on the experimental culture of the red seaweed Gracilaria spp. in Malaysia Malaysia: Department of Fisheries, Ministry of Agriculture; 1986.
- [Google Scholar]
- Algae as nutrition, medicine and cosmetic: The forgotten history, present status and future trend. World J Pharm Pharm. 2017;6:1934-59.
- [CrossRef] [Google Scholar]
- Effects of sulfate group in red seaweed polysaccharides on anticoagulant activity and cytotoxicity. Carbohydr Polym. 2014;101:776-85.
- [CrossRef] [PubMed] [Google Scholar]
- Consolidated bioprocessing of wastewater cocktail in an algal biorefinery for enhanced biomass, lipid and lutein production coupled with efficient CO2 capture: An advanced optimization approach. J Environ Manage. 2019;252:109696.
- [CrossRef] [PubMed] [Google Scholar]
- Recent progress of algae and blue-green algae-assisted synthesis of gold nanoparticles for various applications. Bioprocess Biosyst Eng. 2019;42:1-15.
- [CrossRef] [PubMed] [Google Scholar]
- Algal biorefinery: A sustainable approach to valorize algal-based biomass towards multiple product recovery. Bioresour Technol. 2019;278:346-59.
- [CrossRef] [PubMed] [Google Scholar]
- A review of thermochemical conversion of waste biomass to biofuels. Energies. 2022;15:6352.
- [CrossRef] [Google Scholar]
- Microdiesel: Escherichia coli engineered for fuel production. Microbiology (Reading). 2006;152:2529-36.
- [CrossRef] [PubMed] [Google Scholar]
- Biodiesel from Plant resources-sustainable solution to ever increasing fuel oil demands. J Sustain Bioenergy Syst. 2013;3:163-70.
- [CrossRef] [Google Scholar]
- Production of algal biodiesel from marine macroalgae Enteromorpha compressa by two step process: Optimization and kinetic study. Bioresour Technol. 2013;128:392-400.
- [CrossRef] [PubMed] [Google Scholar]
- An overview of algae biofuel production and potential environmental impact. Environ Sci Technol. 2012;46:7073-85.
- [CrossRef] [PubMed] [Google Scholar]
- The future oil. 2009. Available from: https://www.thescientist.com/uncategorized/future-oil-44421 [Last accessed on 2013 Dec 06]
- [Google Scholar]
- Life cycle assessment of macroalgae cultivation and processing for biofuel production. J Clean Prod. 2014;75:45-56.
- [CrossRef] [Google Scholar]
- Managing R&D in renewable energy: Biofuel vs alternate technologies. AgBioForum. 2010;13:375-31.
- [Google Scholar]
- Reliability analysis of offshore wave energy and seaweed farming system United States: David Publishing Company; 2017.
- [Google Scholar]
- A review on processing technology for biodiesel production. Trends Appl Sci Res. 2015;10:1-37.
- [CrossRef] [Google Scholar]
- Comparison of direct transesterification of algal biomass under supercritical methanol and microwave irradiation conditions. Fuel. 2012;97:822-31.
- [CrossRef] [Google Scholar]
- Characterization of microalga Scenedesmus sp. ISTGA1 for potential CO2 sequestration and biodiesel production. Renew Energy. 2015;74:774-81.
- [CrossRef] [Google Scholar]
- Production of biofuels from microalgae-a review on cultivation, harvesting lipid extraction, and numerous applications of microalgae. Renew Sust Energy Rev. 2018;94:49-68.
- [CrossRef] [Google Scholar]
- Microalgae for biodiesel production and other applications: A review. Renew Sustain Energy Rev. 2010;14:217-32.
- [CrossRef] [Google Scholar]
- Heterotrophic/mixotrophic cultivation of oleaginous Chlorella vulgaris on industrial co-product. Algal Res. 2012;1:40-8.
- [CrossRef] [Google Scholar]
- Bioethanol production from Chlorella vulgaris biomass cultivated with plantain (Musa paradisiaca) peel extract. Adv Biosci Biotechnol. 2017;8:478.
- [CrossRef] [Google Scholar]
- Production of high concentrations of bioethanol from seaweeds that contain easily hydrolyzable polysaccharides. Process Biochem. 2011;46:2111-6.
- [CrossRef] [Google Scholar]
- Converting carbohydrates extracted from marine algae into ethanol using various ethanolic Escherichia coli strains. Appl Biochem Biotechnol. 2011;164:878-88.
- [CrossRef] [PubMed] [Google Scholar]
- Bioethanol production from Gracilaria verrucosa, a red alga, in a biorefinery approach. Bioresour Technol. 2013;135:150-6.
- [CrossRef] [PubMed] [Google Scholar]
- Seasonal variation in Laminaria digitata and its impact on biochemical conversion routes to biofuels. Bioresour Technol. 2011;102:9976-84.
- [CrossRef] [Google Scholar]
- Chemo-enzymatic saccharification and bioethanol fermentation of lipid-extracted residual biomass of the microalga, Dunaliella tertiolecta. Bioresour Technol. 2013;132:197-201.
- [CrossRef] [PubMed] [Google Scholar]
- Study on saccharification techniques of seaweed wastes for the transformation of ethanol. Renew Energy. 2011;36:84-9.
- [CrossRef] [Google Scholar]
- Managing R&D risk in renewable energy: Biofuels vs. Alternate technologies. AgBioForum. 2010;13:375-81.
- [Google Scholar]
- Mooring analysis for large offshore aquaculture ocean plantation floating structure. Ocean Coast Manag. 2013;80:80-8.
- [CrossRef] [Google Scholar]
- Uptake and release of nitrogen by the macroalgae Gracilaria vermiculophylla (Rhodophyta) J Phycol. 2006;42:515-25.
- [CrossRef] [Google Scholar]
- Design and model testing of offshore aquaculture floating structure for seaweed oceanic plantation. Biosci Biotechnol Res. 2012;9:477-94.
- [CrossRef] [Google Scholar]
- Open sea floating structure for offshore aquaculture oceanic macro algae cultivation, PI 2013001596 Malaysia: WIPO; 2013.
- [Google Scholar]
- Biodiesel from Microalgae: A critical evaluation from laboratory to large scale production. Appl Energy. 2013;103:444-67.
- [CrossRef] [Google Scholar]
- State of the art and challenges for multi-trophic offshore aquaculture. Front Mar Sci. 2018;5:165.
- [CrossRef] [Google Scholar]
- Study the performance of biofuel from B5 and Laminaria seaweed on engine test bed for marine transportation. Biosci Biotech Res Asia. 2016;13:1885-93.
- [CrossRef] [Google Scholar]
- Sea farms as a safe and sustainable food source: An investigation on use of seaweeds for liver detoxification and reduced DNA damage in Lates calcarifer (Bloch, 1790) In: Ksibi M, Dhia HB, Khélifi N, Kallel A, eds. Recent advances in environmental science from the euro-Mediterranean and surrounding regions (2nd ed). Cham: Springer; 2019.
- [Google Scholar]
- New insight into marine biotechnology: Carrageenans chemical features and acetylcholinesterase (AChE) inhibition activity of two edible seaweeds of the genus Kappaphycus In: Ksibi M, Negm A, Hentati O, Ghorbal A, Sousa A, Rodrigo-Comino J, eds. Recent advances in environmental science from the euro-Mediterranean and surrounding regions (2nd ed). Cham: Springer; 2019.
- [Google Scholar]
- Biofuels from algae: Challenges and potential. Biofuels. 2010;5:763-84.
- [CrossRef] [PubMed] [Google Scholar]
- Macro algae species and their potential application as raw material for biotech products. Biosci Biotech Res Asia. 2015;12:13-7.
- [CrossRef] [Google Scholar]
- Monitoring seaweed aquaculture in the Yellow Sea with multiple sensors for managing the disaster of macroalgal blooms. Remote Sens Environ. 2019;231:111279.
- [CrossRef] [Google Scholar]
- Review of the status and developments in seaweed farming infrastructure. J Marine Sci Eng. 2022;10:1447.
- [CrossRef] [Google Scholar]







