Translate this page into:
Emerging contaminants at trace levels of pesticides perturbs biomolecules in different organs in mice: Role of peroxisome proliferator-activated receptor-alpha

*Corresponding author: Momoh Audu Yakubu, Department of Environmental and Interdisciplinary Sciences, Texas Southern University, Houston, Texas, United States. momoh.yakubu@tsu.edu
-
Received: ,
Accepted: ,
How to cite this article: Gonnabathula PK, Yakubu MA. Emerging contaminants at trace levels of pesticides perturbs biomolecules in different organs in mice: Role of peroxisome proliferator-activated receptor-alpha. Am J Biopharm Pharm Sci. 2024;4:1. doi: 10.25259/AJBPS_17_2023
Abstract
Objectives:
Information is lacking on the consequences of chronic exposure to emerging contaminants at environmentally relevant (trace concentrations) on biomolecules. Environmental exposure to these chemical mixtures happens at trace concentrations and at multiple molecular interactions. The consequences of trace concentrations of multiple pesticides (MPs) on the regulation of selected biomolecules nitric oxide (NO), thiols, superoxide dismutase (SOD), and glutathione S-transferase (GST) in the tissues from wild type (WT) and genetically deficient- peroxisome proliferator-activated receptor-alpha (PPARα) knockout (Null) mice were investigated.
Material and Methods:
Mice were exposed to trace concentrations of MPs: Atrazine, dieldrin, endrin, endosulfan, and anthracene (1–100 ng/L) in drinking water for 6 weeks. Organs were collected and homogenized; NO, protein and non-protein thiol levels, as well as SOD and GST activities were determined.
Results:
Differential and organ selective effects of the treatments were observed in the WT and PPARα knockout. Increased NO levels were observed in the organs from WT with limited increase in the kidney (Null). SOD activity was decreased in the organs from the WT and was increased in the PPARα knockout when compared to the control. Thiol level was significantly increased in the heart and spleen in the WT and in the heart of the PPARα knockout mice when compared to the control. Non-protein thiol concentration was reduced in the heart and kidney (WT) and reduced in the liver of the PPARα knockout when compared to the control. GST activity was significantly decreased in the liver and spleen (WT) and was significantly elevated in all organs in the PPARα knockout mice when compared to the WT.
Conclusion:
The low concentrations of MPs may have caused selective dysregulation of biomolecules in different organs of the body. These effects observed may be influenced by genetic status such as in PPARα deficiency. These results present a scenario that implicates nanoconcentrations of series of organic contaminants that can cause cellular and molecular dysregulations of biomolecules precipitating toxicity and pathology that can be a threat to human health. Further, investigation into the molecular mechanism(s) and signaling pathway(s) implicated in these dysregulations is warranted.
Keywords
Emerging contaminants
Peroxisome proliferator-activated receptor-alpha (PPARα)
Multiple pesticides
Nitric oxide
Superoxide dismutase
Oxidative stress
Glutathione S transferase
Thiol
and Non-Protein Thiol
Antioxidant System
INTRODUCTION
For decades, emerging contaminants have been detected in the environmental matrices. However, their occurrences and significance are only given priority at high concentrations. Their fate, behavior, and (eco) toxicological effects are not well documented at very low concentrations. Thus, it is very difficult to estimate and interpret the consequences of very low levels of these contaminants on long-term environmental exposure.[1-6] Emerging contaminants include but are not limited to water disinfectant byproducts, gasoline additives, manufactured nanoparticles, pesticides, herbicides, ultra-violet filters, human, and veterinary pharmaceutical products.[1-3,7-14] Urbanization, modern lifestyle conveniences, industrialization, high emissions from transportation, industrial discharges, effluents from water, waste treatment plants, as well as increased use of agricultural chemicals, natural processes, and disasters are constantly contributing to environmental contaminations and pollutions. With advances in instrumental analysis of environmental chemicals and toxicants, several chemicals are being detected and reported at trace levels from environmental matrices that were not possible or detected before. The presence of these emerging contaminants of concern (ECC) in the ecosystem can find their way into biological systems, causing dysregulation of biomolecules, altering functions, and resulting in pathologies. The major concern about these contaminants in the human environment is their long-term consequential health effects. However, information is lacking on the related health effects of these chemical mixtures following chronic exposure at trace concentrations and at multiple molecular interactions. Although, substantial insights into their individual toxic reactions and/or effects are well known when biological systems are exposed to them at higher (toxic) concentrations.[15-20] Yet, the effects of these chemicals at trace concentrations which are akin to the environmentally relevant and detected concentrations[4-6,14] on diverse biomolecular processes and defense systems are lacking.
Exposure of biological systems to xenobiotics induces multifactorial effects ranging from activation of xenobiotic metabolizing enzymes (XMEs) to activation of processes that can activate generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) causing oxidative and nitrative stresses, reducing antioxidant defense systems through depletion of glutathione (GSH), interfering with some biologically essential metals, cofactors, inhibiting and/or binding to sulfhydryl dependent enzymes, structural proteins, and/or interferes with antioxidant enzymes’ activities. Thus, increasing the susceptibility of cells to reactive oxidative intermediate attacks and alters membrane integrity, fatty acid compositions, antioxidant status, and cellular dysregulation.[15,16,19,21-27] Under physiological conditions, ROS are cleared from the cells by the actions antioxidant enzymes – superoxide dismutase (SOD), catalase (CAT), thiols, or GSH.[28-30] Fortunately, under normal physiological conditions, the balance between free radical generation in tissues and endogenous antioxidants enzymes’ activities prevents oxidative stress from building up.[31-35] Thus, cellular health depends on the maintenance of this balance. CAT, SOD, glutathione S-transferases (GSTs), and glutathione peroxidase are the main endogenous antioxidant enzymes commonly found in living tissues. These enzymes (SOD, GST, and CAT) work in concert to convert super oxides and peroxides into harmless products of water and oxygen.[19,20,29,35] These antioxidant enzyme systems are targets for xenobiotic attacks and dysregulations contributing to various pathologies.
The XMEs are regulated and activated at the transcriptional level through activation of xenobiotic sensing receptors, including peroxisome proliferator-activated receptor-alpha (PPARα), and nuclear factor-E2-related factor 2 among others.[31,36-50] This transcription factors act as biosensors in response to the presence of xenobiotics and induces expression of genes for enzymes responsible for xenobiotic metabolism and biotransformation as well as antioxidant enzymes.[47-50] PPARα, the first peroxisome proliferator-activated receptor (PPAR, later defined as PPARα [NR1C1]), was identified in 1990[38] and plays very important roles in homeostasis.[49,50] PPARs have been implicated in the regulation of cellular metabolism and in the modulation of inflammatory responses linked to liver fibrogenesis and oxidative stress.[44,48-53] Given the vast processes PPARα regulates, the absence of PPARα can lead to dysregulation of vital biomolecular processes and dysfunctions.
We have investigated the effects of trace concentrations of multiple pesticides (MPs) in the regulation of selected biomolecules in tissues from wild type (WT) and PPARα knockout mice. This study is designed to enhance our understanding of the consequences of exposure to these contaminants in normal mice and genetically deficient-PPARα (knockout) mice.
MATERIAL AND METHODS
Chemicals and reagents
2-Bromo-2-chloro-1,1,1-trifluroethane 99% (Anesthesia), atrazine, dieldrin, endrin, endosulfan, anthracene, 0.1% N-(1-naphthyl) ethylene diamine dihydrochloride, phosphoric acid, sulfanilic acid, sodium nitrite (NaNO2), epinephrine, ethylenediaminetetraacetic acid, 1-chloro-2,4,-dinitrobenzene (CDNB), hydrogen peroxide, trichloroacetic acid, 5’-5’-dithiobis-(2-dinitrobenzoic acid) (DTNB), reduced GSH, phosphate buffer, potassium chloride (KCl) solution, potassium phosphate buffer, carbonate buffer, and all other chemicals used were of analytical grade. These chemicals were bought from Sigma Aldrich, St. Louis MO, USA.
Experimental animals
WT and PPAPα knockout female mice (from Harlon, Houston) were selected and randomly divided into control (n = 4) and experimental WT groups (n = 4). Similarly, the PPARα knockout mice were divided into control (n = 4) and experimental groups (n = 4). The control groups received tap water while experimental groups were supplied with drinking water containing MPs: Atrazine, dieldrin, endrin, endosulfan, and anthracene at a concentration of 1–100 ng/L ad libitum for 6 weeks. The dose selected for this study is consistent with detected concentrations within the environment[3,10,11,14,54,55] and within the maximum residue limit (MRL) by environmental protection agency (EPA).[12] Furthermore, mice were fed a commercial chow diet and subjected to a photoperiod of 12 h light-dark cycle. Mice were treated humanely according to the National Institute of Health Guideline on the care and treatment of experimental animals and the study protocol was approved by the Texas Southern University Institutional Animal Care and Use Committee (IACUC).
Organs, blood, and tissue collection and preparation for biochemical assays
At the end of the six weeks of treatments, mice were anesthetized with 2-bromo-2-chloro-1, 1, 1-trifluroethane 99%, and organs (heart, spleen, liver, kidney, and brain) harvested. Organs were rinsed with ice-cold 1.15% KCl, minced with scapple, and homogenized in ice-cold 0.1 M phosphate buffer, pH 7.4 using Teflon homogenizer. The resultant homogenate was centrifuged at 10,000 rpm (40°C) for 10 min. The post mitochondrial fractions, collected, processed, and stored at −80°C until needed for biochemical assays.
Biochemical assays
Nitric oxide (NO) assay
The effects of treatments on NO concentrations in the post-mitochondrial fractions of the organ’s homogenates were determined by modified Griess Reagent Assay.[56,57] Briefly, into the 96 well plate was added 100 μL of tissue samples and to this was added 100 μL of Griess reagent mixture comprising solution A and B (1:1; v/v) (solution A [0.1% N-(1-naphthyl) ethylenediamine dihydrochloride] and B [1% sulfanilamide in 2.5% H3PO4]). The mixture was incubated at room temperature for 20 min, and absorbance read at 480 nm using Bio-Tek ELx808 Absorbance Microplate Reader, Winooski, Vermont, U.S.A. The concentration of nitrite in the sample was determined from NO standard curve constructed using NaNO2 and NO concentration was expressed as μmol nitrite.[56,57]
SOD activity determination
The effects of treatment on the activity of SOD in samples were determined by the modified method of Misra and Fridovich[58] as modified and reported by Oyagbemi et al.[36] In the presence of toxicants, super oxide generated by xanthine oxidase causes oxidation of epinephrine to adrenochrome. SOD inhibits the auto-oxidation of epinephrine to adrenochrome. Adrenochrome generation is increased by super oxide free radical chain reaction; hence SOD produces inhibitory effect on its generation. SOD activity can be estimated by measuring absorbance at 480 nm which denotes either an increase or decrease in activity of SOD. In general, increased absorbance indicates decrease SOD activity and vice versa. Briefly, 50 mg of epinephrine was dissolved in 100 mL of distilled water and acidified with 0.5 mL concentrated hydrochloric acid. This preparation prevents oxidation of epinephrine and is stable for 4 weeks. 30 μL sample was added to 2.5 mL 0.05 M carbonate buffer (pH 10.2) followed by the addition of 300 μL of 0.3 mM adrenaline. The absorbance at 480 nm was monitored every 30 s for 150 s.
Protein thiol content determination
Thiol content was determined using Ellman’s reagent DTNB[59,60] as modified by Oyagbemi et al.,[36] The procedure is based on the reaction of thiol with DTNB to give the mixed disulfide and 2-nitro-5-thiobenzoic acid (TNB), which is quantified by the absorbance of the anion (TNB2-) at 412 nm using Bio-Tek ELx808 Absorbance Microplate Reader and to determine the molar concentration of thiols based on the molar extinction of reduced Ellman’s reagent (13,600 M/cm), that is, divides the absorbance at 412 nm by 13,600, which gives molar non-protein thiol concentration.[36,59]
Non-protein thiol content determination
Non-protein thiol concentration was determined by Ellman’s reagent DTNB method and to determine the molar concentration of non-protein thiols based on the molar extinction of reduced Ellman’s reagent (13,600 M/cm), that is, divides the absorbance at 412 nm by 13,600, which gives molar thiol concentration.[36,59] This data was used to plot the graph of effects of emerging contaminants on the molecular concentration of non-protein thiol in the sample.
Estimation of GST activity
GST activity was determined by the reaction that involves the conjugation of CDNB with reduced GSH following the original assay of Habig et al.,[61] as modified by Oyagbemi et al.[36] Following the reaction, changes in absorbance at 340 nm were read by ELx808 Microplate Reader. One unit of enzyme will conjugate 10.0 nmol of CDNB with reduced GSH per minute at 25°C. Changes in absorbance per minute were converted into micromoles (μM) of CDNB conjugated per min per mL of sample using the extinction coefficient of the resulting 5-(2, 4-dinitrophenyl) – GSH: E340 nm = 9.6 nM/cm.[61]
Statistical analysis
Results are presented as mean ± standard deviation (SD) and were analyzed by analysis of variance (ANOVA) comparing results from control to treated groups in different organs within the WT and PPARα knockout. The results from the WT were correlated to that of PPARα knockout to determine the influence of PPARα knockout in changes observed. P = 0.05 was considered significant.
RESULTS
NO production
Figure 1 shows the effects of treatment of WT (a) and PPARα (b) knockout mice with trace concentrations of a selected mixture of emerging contaminants on NO levels in different organs. The basal level of NO was significantly higher in the PPARα knockout compared to the WT mice. Treatment of WT mice with nanoconcentrations of MPs significantly elevated NO levels in all the organs when compared to the control [Figure 1a]. In the PPARα knockout mice, treatments showed differential effects on the levels of NO with no change observed in the heart and liver, but increased levels in the kidney and brain with reduced levels in the spleen [Figure 1b].
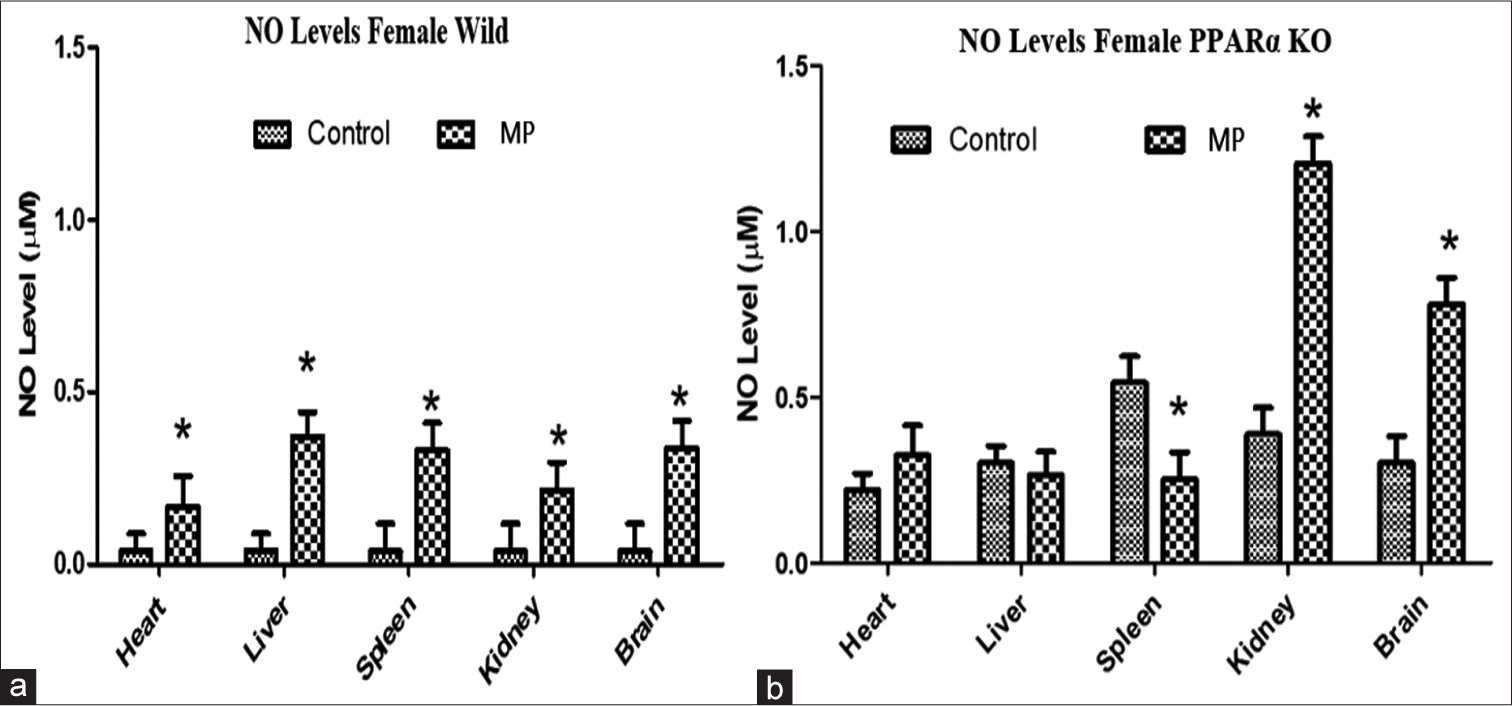
- Effects of treatment of (a) wild type and (b) peroxisome proliferator-activated receptor-alpha (PPARα) knockout mice with trace concentrations of selected mixture of emerging contaminants on nitric oxide levels (μM) in different organs. Results are expressed as mean ± standard deviation, *P = 0.05, two-way analysis of variance (n = 4). NO: Nitric oxide, KO: Knockout, MP: Multiple Pesticides.
SOD activity
Figure 2 shows the effects of treatment on SOD activity in organs from WT (a) and PARα knockout mice (b). Differential basal SOD activity was observed in the organs from the WT and the PPARα knockout mice, while basal SOD activity was significantly higher in the heart and liver and lower in the kidney and brain with no change in the spleen of the WT compared to the knockout. Treatment of WT with trace concentrations of emerging contaminants significantly reduced SOD activity in the heart, liver, and brain (by 86%, 50%, and 36%, respectively) with no changes in spleen and kidney [Figure 2a]. While in the PPARα knockout mice, treatment significantly increased SOD activity in the heart, liver, and spleen (88%, 87%, and 29%, respectively) with reduction in kidney (41%) and brain (29%) [Figure 2b]. The effects of PPARα knockout on basal SOD activity is an indication of the fact that PPARα may be involved in the maintenance of SOD activities to a varying degree in different organs. Consistent with expectation, MP treatment led to a reduction in SOD activity in the heart, liver, and brain with no change in the spleen and kidney in the WT. In the PPARα knockout, treatment produced opposite effects (to the effects observed in the WT) by significantly increasing SOD activities in the heart, liver, and spleen; and reducing the activities in the kidney and brain.
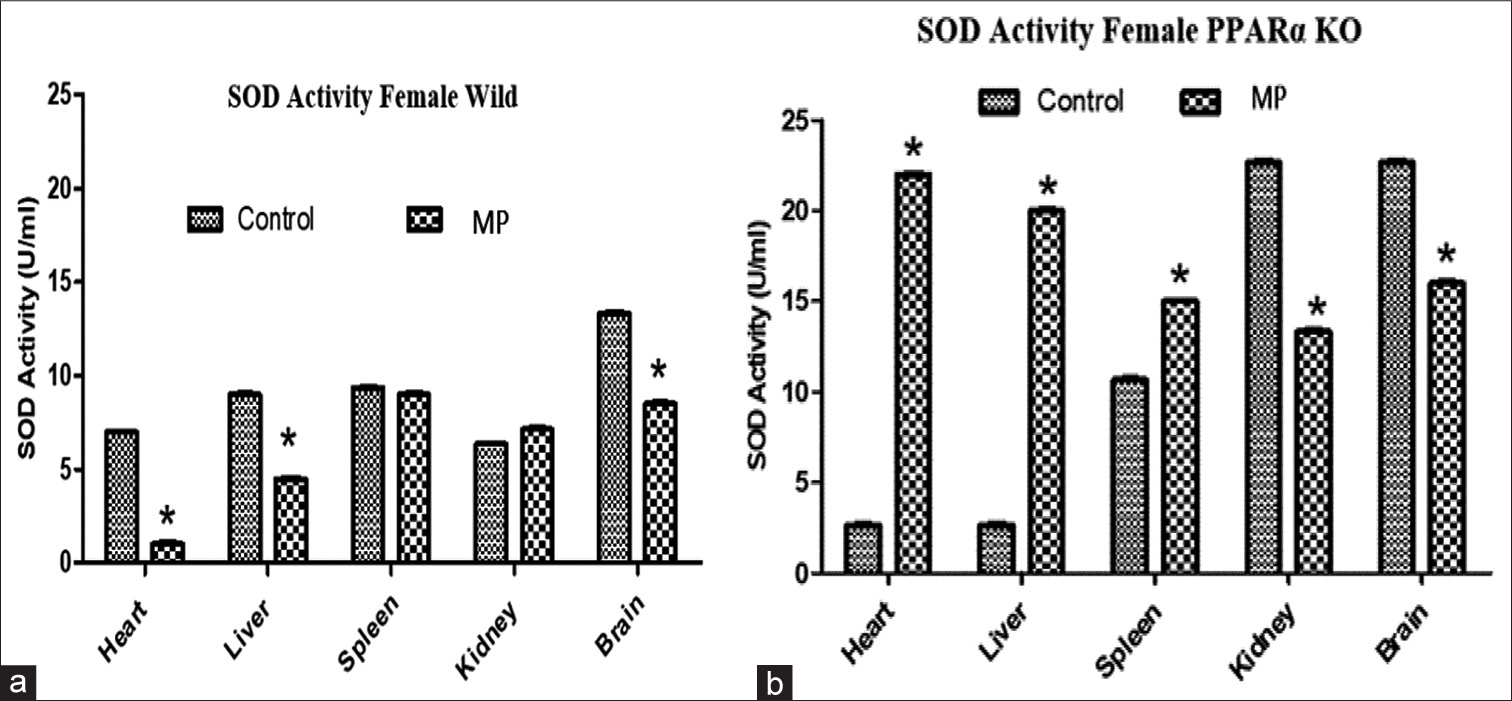
- Effects of treatment on superoxide dismutase (SOD) activity in organs from (a) wild type and (b) peroxisome proliferator-activated receptor-alpha knockout mice. Results are expressed as mean ± standard deviation, *P = 0.05, analysis of variance (n = 4).
Thiol levels
Figure 3 shows the effects of treatment with nanoconcentrations of ECC on thiol levels in organs from WT and PPARα knockout mice. The basal levels of thiol in the different organs from both WT and PPARα knockout mice seem to be similarly distributed. Thiol levels were significantly increased in the heart (21%), spleen (23%), and kidney (10%), with reduction in the liver (21%) and brain (11%), but no change was observed in the kidney following treatment in the WT [Figure 3a]. In the PPARα knockout mice, thiol level was increased in the heart (23%) and reduced in the brain (10%) similarly to that observed in the WT, but no changes were observed in the liver, spleen, and kidney [Figure 3b].

- Effects of treatment with nanoconcentrations of emerging contaminants of concern on thiol levels in organs from (a) wild type and (b) peroxisome proliferator-activated receptor-alpha (PPARα) knockout mice. Results are expressed as mean ± standard deviation, *P = 0.05, analysis of variance (n = 4).
Non-protein thiol
Figure 4 shows the effects of treatment with trace concentrations of ECC on non-protein thiol in organs from WT and PPARα knockout mice. The basal levels of non-protein thiol in the different organs from both WT and PPARα knockout mice are similarly distributed. Non-protein thiol levels in the WT were significantly reduced in the heart (8%) and kidney (7%) with no change in liver and spleen but a slight increase in brain (4%) [Figure 4a]. In the PPARα knockout mice, we observed a slight increase in the heart with reduction in the liver and brain [Figure 4b].

- Effects of treatment with trace concentrations of emerging contaminants of concern on non-protein thiol in organs from (a) wild type and (b) peroxisome proliferator-activated receptor-alpha (PPARα) knockout mice. Results are expressed as mean ± standard deviation, *P = 0.05, analysis of variance (n = 4).
GST activity
Figure 5 shows the effects of treatment with nanoconcentration of ECC on GST activity in organs from WT and PPARα knockout mice. The basal GST activities were reduced in the liver, spleen, and brain from the PPARα knockout compared to the WT mice. In the WT, treatments with nanoconcentrations of MPs differentially regulated GST activities, significantly increasing levels in the liver and kidney while reducing the levels in the spleen and brain when compared to the control [Figure 5a]. In the PPARα knockout mice, GST activity was significantly elevated in all the organs; in the heart (35%), liver (39%), spleen (20%), kidney (49%), and brain (30%) when compared to the control [Figure 5b].
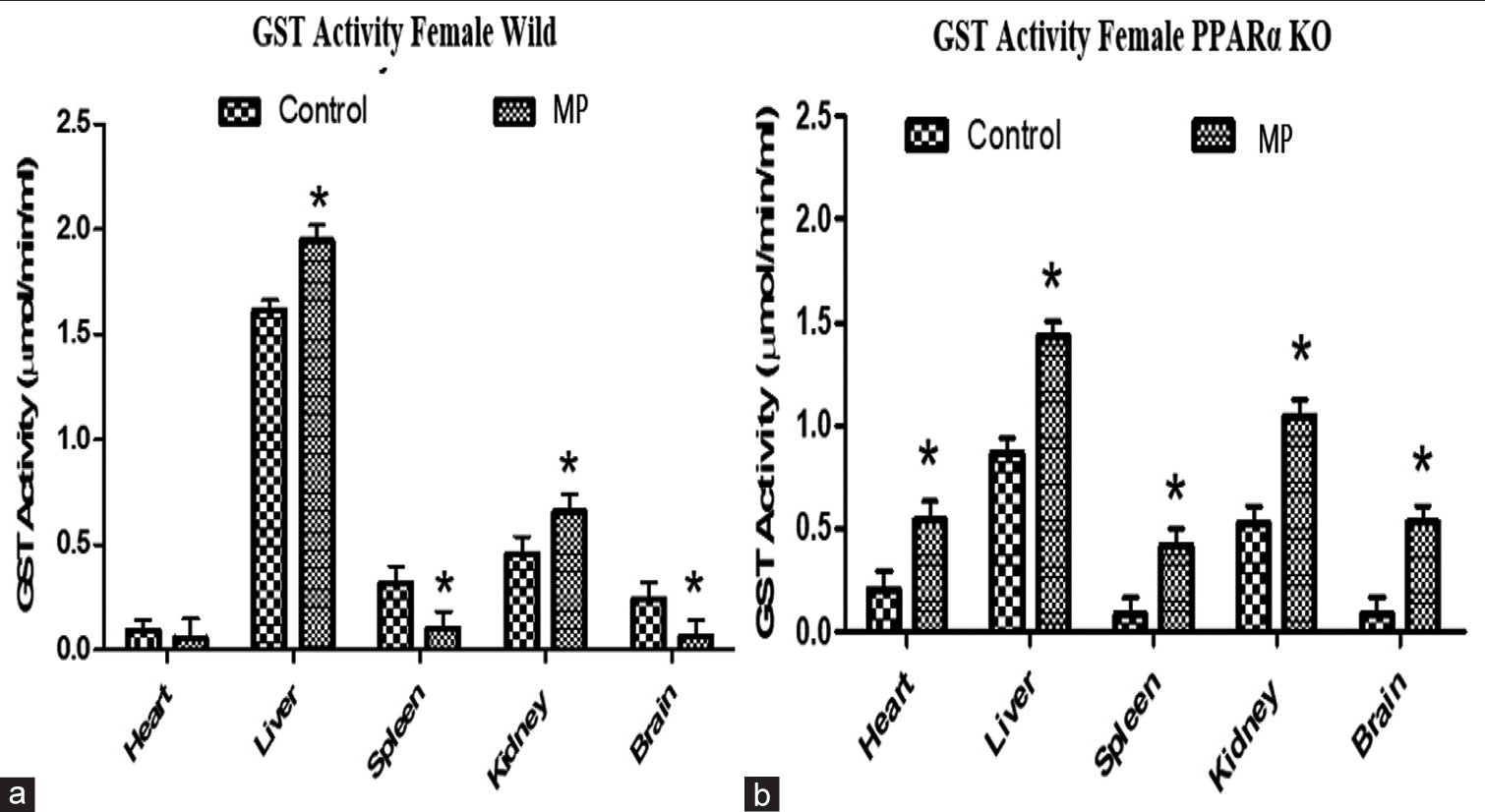
- Effects of treatment with nanoconcentration of emerging contaminants of concern (ECC) on glutathione S-transferase activity in organs from (a) wild type and (b) peroxisome proliferator-activated receptor-alpha (PPARα) knockout mice. Results are expressed as mean ± standard deviation, *P = 0.05, analysis of variance (n = 4). GST: Glutathione S transferase.
DISCUSSION
Foods for human consumption are increasingly being contaminated with emerging contaminants derived particularly from household use of pesticides, disinfectants, detergent by-products, pharmaceuticals, personal care products, and several other human and industrial activities. These contaminants of concern are widely distributed and persistent in the environment, they leach, and wash into our foods and water.[13,14,50,60,62] The chemicals taken up by marine habitats bioaccumulate in sea foods, predator birds, as well as in humans. They have been associated with major health concerns including antibiotic resistance, endocrine disruption, infertility, diabetes, cardiovascular diseases, cancer, and developmental disabilities at high concentrations.[3,5,6,26,27,35,63-69] However, emerging contaminants at low concentrations and in multiple interactions could have potential ecological and negative public health impact. However, data from studies on their risks and potential adverse health effects at low concentrations in biological systems are limited or are lacking.[3,7,10,65] This is the first study to our knowledge that have evaluated and reported the effects of trace concentrations (environmentally relevant concentrations) of mixtures of multiple pesticides on biomolecules especially antioxidant enzymes and molecules in different organs of WT and PPARα null mice.
We have investigated the effects of treatment of WT as well as PPARα knockout mice with low concentrations of several MPs found in our environment, water, and food products to determine their effects on antioxidant enzymes’ status and activities in different organs from the mice. The effects in WT mice were compared with the PPARα knockout mice to further understand the effects of these environmental contaminants on animals that may be genetically compromised. PPARα is a known transcription factor that plays an important role in the regulation of antioxidant enzymes and activities to help maintain metabolic stability. In this study, we have found that (a) basal NO levels in the WT were lower compared to the PPARα knockout; treatment with low concentrations of emerging contaminants increased NO levels in all organs of WT mice; while in the PPARα knockout mice, treatment resulted in selective increase in NO levels in kidney and brain with a decrease in spleen. (b) Basal SOD activity was lower in the heart and liver of PPARα knockout mice compared to the WT and treatment decreased SOD activities in the heart, liver, and brain (WT mice) with no change in spleen and kidney. In the PPARα knockout mice, treatment resulted in increased SOD activities in the heart, liver, and spleen with reduction in the kidney and brain. (c) Basal thiol level was significantly higher in WT compared to the PPARα knockout mice. Treatment resulted in increased thiol contents in the heart, kidney, and spleen (WT mice) with reductions in the liver and brain, and no change in the kidney. Thiol level was increased in the heart, but no changes were observed in the liver, spleen, and kidney, with a slight decrease in the brain following treatment (PPARα knockout). (d) Non-protein thiol content was low in all organs except the liver (PPARα knockout) compared to the WT. Treatment resulted in a reduction in non-protein thiol level in the heart and kidney with no change in the liver, spleen, and brain in the WT; while in the PPARα knockout mice, treatment resulted in a significant reduction in non-protein thiol in the liver and brain as well as an increase in the heart with no change in kidney and spleen. (e) Differential reduction in basal GST activity was observed in knockout mice liver, spleen, and brain with no change in the kidney when compared to the WT. Treatment with the MPs significantly increased GST activity in the liver and kidney with a decrease in spleen and brain and no change in the heart in the WT compared to the control. In the PPARα knockout mice, GST activity was significantly elevated in the heart, liver, spleen, kidney, and brain when compared to the control.
Exposure of the body and ecosystem to MPs at nanoconcentrations may induce a broad range of biochemical, physiological, and other dysfunctions possibly through induction of oxidative stress and/or dysregulation of antioxidant systems. Antioxidative enzyme systems are actively involved in maintaining the oxidant/antioxidant balance and homeostasis by detoxification of xenobiotics in the body. The maintenance of cellular homeostasis depends on defense systems which are essential for overcoming the deleterious effects of radicals generated.[5,14,18-20,29,39,40,54,64-81] However, depletion of the cellular antioxidant pool is characterized by (a) increased ROS and RNS production; (b) depletion of free-radical scavengers (GSH, thiol, and others), and cellular antioxidants (largely GSH); and (c) inhibition of the activity of enzymes such as SOD, GSH- reductase, and GSH-transferase. All of these contribute significantly to the metabolism and detoxification of ROS. Hence, grave consequences may result from the destabilization of controlled detoxification of toxicants within the bodies of living organisms. Consistent with this, we have reported in the present study that treatment with MPs at trace concentrations resulted in the dysregulation of redox systems. This suggests that deleterious effects of low concentrations of MPs possibly interacting at multiple molecular sites in the body to cause dysregulation of homeostasis through alteration of antioxidant biomolecules.
ROS have several effects on cellular functions. At low concentrations, they modify and fine-tune intracellular signaling like in immune modulation and signaling, wound healing among others. Cellular processes and toxicants induced ROS production are catalyzed by SOD to H2O2 on which CAT acts to degrade into H2O and O2.[29,56,64,71,82,83] Oxidative and nitrative stress developed in response to toxicants plays important roles in the disruption of biomolecules such as enzymes, proteins, deoxyribonucleic acid, and hormones as well as disrupting signaling transduction pathways. This, in turn, can lead to pathogenesis of animal and human diseases. From a pathological perspective, oxidative stress can contribute to multiple diseases including autoimmunity and cardiovascular disease, which accelerate the normal aging process, atherosclerosis, inflammation, and cancer.[6,27,35,39,71,74,82] Despite antioxidant protections afforded by cellular redox systems networks in biological processes, the presence of toxicants can lead to disruption of antioxidant enzymes. Consequently, such disruption can lead to increased intracellular ROS/RNS levels as we have observed in this study. The increased oxidative and nitrative radicals generated can lead to the production of cellular destructive agent. These effects are usually checked and eliminated by a complex antioxidant (enzymatic and non-enzymatic) system under normal conditions.[31,32,35] The enzymatic antioxidant system constitutes the first line of defense, and then reduced thiols, followed by low-molecular-weight antioxidants and by a broad range of products from dietary sources. NO, a chemical messenger and free radical by-product of reactions catalyzed by NO synthase (NOS) enzymes, is essential for numerous physiologic processes, but is also a pro-oxidant capable of contributing to oxidative/nitrative stress and damaging an array of cell types.[29,35,42,70,71,75] The persistent perturbations of biological systems by ROS/RNS lead to inflammatory processes which could lead to major pathologies such as diabetes, hypertension, and cancer.[26,27] Treatments with MPs resulted in a significant increase in NO levels more than 500% compared to the control in WT. This degree of increase in NO is an indication of a response consistent with nitrative stress and a high possibility of the production of peroxynitrite (ONOO−), one of the most toxic radical species. The peroxynitrite can be formed by the heighten generation of oxidative stress (ROS) along with the high concentration of NO as a consequent of xenobiotic activation of inducible NO (iNOS) a natural target of toxicants.[35,43,63,70,82,84] In the PPARα knockout, treatment had limited effects on the fluctuation of NO levels as the increase was not as high as observed in the WT. This buffering effects observed in PPARα knockout mice, which is surprising given the antioxidant effects of PPARα. PPARα is known to play a role in the regulation of antioxidant genes and enzymes of which NOS is one of them.[37,56,57,85] It has been reported that PPARα agonists can enhance the antioxidant and anti-inflammatory defense systems by upregulating the expression of antioxidant enzymes and inhibition of nuclear factor kappa B (NF-κB) activity following cellular damage and injury.[46,85,86] However, in the absence of PPARα gene, other PPAR isoenzymes (β, γ/δ) may be activated by the MPs, leading to the tight control of the fluctuations in the level of NO as observed in PPARα knockout. The source of the NO increase is possibly through activation of iNOS which is a target for toxicants and pathological conditions.[30,82,87,88] Overexpressed or dysregulated iNOS activation has been implicated in numerous pathologies including sepsis, cancer, neurodegeneration, and various types of inflammation and pain.[70,75] This could be an indication that this PPAR isoform may be pro-iNOS (activation) as such it’s absent resulted in the tight regulation of NO levels compared to the WT. It is well known that PPARs regulate the production of NO via inducible (iNOS) as well as eNOS dependent enzymes.[56,57,89-91] Consistent with our observation and speculation is the reported PPARγ agonists decreasing NF-κB, interferon γ- or lipopolysaccharide-induced NO production,[84,90,92,93] and iNOS expression[84,87,93,94] Such PPARγ action might completely reprogram the transcription process as reported resulting in the observed effect in this study. With the absence of PPARα gene and its unavailability for activation (phosphorylation), transactivation function is diminished, giving rise to the recruitment of other regulatory pathways, possibly of other PPAR isoforms to regulate the oxidative stress.
GSH is the most abundant intracellular thiol-based antioxidant, prevalent in millimolar concentrations in all living cells. Its function is mainly as a sulfhydryl buffer, GSH also serves to detoxify compounds either through conjugation reactions catalyzed by GST or directly. In the present study, trace concentrations of toxicants increased the activity of GSH in the liver, kidney with reduction in the brain and spleen. The implication of this differential regulation is not known but depends on the organ and its GSH concentrations-relative to antioxidant activities taking place therein. The liver is the organ with the highest metabolic capacity followed by the kidney and possibly high antioxidant enzymes and activities. It could be a major target for these multiple toxicants in the body. Consistent with our observation is the reported chemical injury to the liver and other organs following exposure to xenobiotics.[19,20,24,71,95]
Thiol groups are very pivotal in signaling pathways and homeostasis through oxidation, reduction, and disulfide exchange, which are involved in many physiological functions.[28,74,76] The dysregulation of these processes can result in pathology and have been designated as disease biomarkers.[4-6,10-14,17-96] Thiol or sulfhydryl (–SH) group is a highly active reduced sulfur in biomolecules, present in amino acids such as cysteine proteins and peptides; it is particularly sensitive to redox reactions.[76,77] Hence, thiol is a target for regulation under oxidative stress conditions. Oxidation of cysteine could be the reason behind changes in protein structure and functions or other biomolecules. In the present study, treatment of WT mice resulted in a reduction in thiol levels in the liver and brain with increased level in the spleen, heart, and kidney. In the PPARα knockout, treatment had limited effects on thiol level with slightly reduced levels in the brain and increased level in the heart, similarly to WT mice. The reasons behind the differential effects in these organs are not known. However, it could be due to the different sensitivities of the organs to the generation of oxidative stress following treatment with MPs. Furthermore, it could be due to possible perturbations of biomolecules by MPs at low concentrations as against outright changes induced by high concentrations of single toxicants reported by the previous studies.[19,20,24] The contributions of PPARα to the regulation of thiol levels are not well understood as effects of treatment in PPARα knockout mice abrogated the cellular changes observed in the WT. This may be an indication that PPARα activation might contribute to the changes observed in WT and its absence produced a tighter regulation in the thiol levels. Consistent with this, our observation is the reported possible pro-oxidative effects of some PPAR ligands; some studies suggest that PPAR agonists may also have prooxidative and inflammatory effects.[40,45,46] Furthermore, in the absent of PPARα isoform, other PPAR isotypes γ and β/δ present may be activated to regulate antioxidant defensive responses and maintain the relative changes observed in the WT following treatment with MP.
The main non-protein thiol is the tripeptide GSH, due to its intracellular concentration; it is a very important component of antioxidant defense systems to scavenge ROS.[76,78,79] It was only slightly reduced in the heart and kidney, in PPARα knockout, there was an increase in the heart, liver, and brain. The implications of these effects are not known but may not amount to much significance as the levels in both groups are consistent and do not differ significantly. Furthermore, further investigation is needed to clarify the roles of PPAR isoenzymes in the regulation of the tripeptide GSH in toxicant induce regulation of cellular functions.
Pesticides are known to cause serious ailments in the non-target animal species. The results of our study revealed that trace concentrations of MPs caused serious perturbations in the levels of anti-oxidative enzyme activities (cellular antioxidants: GSH, GST, SOD, and CAT). Our results also showed that PPARα gene plays a significant role in the modulation of these antioxidant enzymes’ activities. In sum, PPARα is an important lipid sensor and regulator of cellular energy-harvesting metabolism and consistent with this important role is the potent genetic proof that reported that PPARα null mice had depressed levels of numerous fatty acid metabolizing enzymes and were unresponsive to the actions of peroxisome proliferating agents.[80] Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishing the pleiotropic effects of peroxisome proliferators.[80] In rodents and humans, PPARα is expressed in numerous metabolically active tissues including liver, kidney, heart, skeletal muscle, and brown fat. The second candidate, PPARγ, is not highly expressed in PPARα WT mouse liver for example; however, feeding PPARα knockout mice high fat diets increased PPARγ expression by ∼4-fold,[81] with a concomitant upregulation of other enzymes regulated by PPARα. PPARγ may compensate for PPARα actions in PPARα knockout mice. As suggested in our present study, PPAR’s regulation of cellular redox states appears to be a highly diversified function that may be dependent on the specific subtype at a particular tissue under different metabolic and stress conditions. We can, therefore, suggest that cellular PPAR signaling can be directly dependent and related to PPAR expression, protein activities, and PPAR interactions with their ligands and coregulators. The three PPAR subtypes regulate cellular biomolecular lipid and energy metabolism in most tissues in the body with overlapping and preferential effects on different metabolic steps depending on the tissue type. The PPAR effects in this study are further complicated by the fact that specific ligands of each PPAR isotypes may display differential potencies and specificities in their role in redox pathways’ regulations. It appears therefore, that in the present study with multiple pesticides, generated oxidative stress may influence individual PPAR isoenzymes’ activity in a tissue specific manner and this calls for further study.
CONCLUSION
Low concentrations of MPs caused selective dysregulation of NO/SOD systems, thiol and non-protein thiol content of the antioxidant components in different body organs possibly due to free radical generation. The effects observed may be influenced by genetic status as in PPARα knockout. However, such effects of PPARα need further investigation as there seems to be no clear-cut role of PPARα in the control of these antioxidant enzyme systems. Possibilities exist that PPARα may not have a dominant control on the regulation of antioxidant enzyme activities. Consistent with this is the fact that the other isoforms of PPARs (γ, β/δ) may still exert control on the antioxidant enzymes in the absence of PPARα gene. This is consistent with the fact that the MPs at low concentration can also activate the other PPARs isoenzymes.
Acknowledgments
This research is supported by Title III (Supplies and Fellowship awarded to PG) under the Award Number P031B090216; Title III, Part B, Historically Black Graduate Institutions (HBGI) (CFDA No. 84.031B).
Author’s contributions
PKG: Contributed to the experimental sample collections, sample preparations, biochemical assays, collation of results, graphing, manuscript draft, and writing. MAY: Conceptualization of the study, experimental design and performing experiments, sample collection, data analysis, and interpretation; manuscript draft, writing, and revision.
Ethical approval
The research/study approved by the Institutional Review Board at Texas Southern University, number 1111, dated July 22, 2016.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Emerging pollutants in the environment: Present and future challenges in biomonitoring, ecological risks, and bioremediation. N Biotechnol. 2015;32:147-56.
- [CrossRef] [PubMed] [Google Scholar]
- Identifying chemicals and mixtures of potential biological concern detected in passive samplers from great lakes tributaries using high-throughput data and biological pathways. Environ Toxicol Chem. 2021;40:2165-82.
- [CrossRef] [PubMed] [Google Scholar]
- Persistent contaminants of emerging concern in a great lakes urban-dominant watershed. J Great Lakes Res. 2022;48:171-82.
- [CrossRef] [Google Scholar]
- Source, transport, and toxicity of emerging contaminants in aquatic environments: A review on recent studies. Environ Sci Pollut Res. 2023;30:121420-37.
- [CrossRef] [PubMed] [Google Scholar]
- Exposure to modern, widespread environmental endocrine disrupting chemicals and their effect on the reproductive potential of women: An overview of current epidemiological evidence. Hum Fertil (Camb). 2019;22:2-25.
- [CrossRef] [PubMed] [Google Scholar]
- Organophosphate pesticide exposures, nitric oxide synthase gene variants, and gene-pesticide interactions in a case-control study of Parkinson's disease, California (USA) Environ Health Perspect. 2016;124:570-7.
- [CrossRef] [PubMed] [Google Scholar]
- Ecotoxic pharmaceuticals, personal care products, and other emerging contaminants: A review of environmental, receptor-mediated, developmental, and epigenetic toxicity with discussion of proposed toxicity to humans. Crit Rev Environ Sci Technol. 2016;46:336-81.
- [CrossRef] [Google Scholar]
- Occurrence, fate and transformation of emerging contaminants in water: An overarching review of the field. Environ Pollut. 2017;231:954-70.
- [CrossRef] [PubMed] [Google Scholar]
- Pharmaceuticals of emerging concern in aquatic systems: Chemistry, occurrence, effects, and removal methods. Chem Rev. 2019;119:3510-673.
- [CrossRef] [PubMed] [Google Scholar]
- Occurrence, distribution, and seasonality of emerging contaminants in urban watersheds. Chemosphere. 2018;200:133-42.
- [CrossRef] [PubMed] [Google Scholar]
- Occurrence of contaminants of emerging concern in aquatic ecosystems utilized by Minnesota tribal communities. Sci Total Environ. 2020;724:138057.
- [CrossRef] [PubMed] [Google Scholar]
- Emerging contaminants: A tutorial mini review. Global NEST J. 2012;14:72-9.
- [CrossRef] [Google Scholar]
- Contaminants of emerging concern in aquatic environment: Occurrence, monitoring, fate, and risk assessment. Water Environ Res. 2020;92:1811-7.
- [CrossRef] [PubMed] [Google Scholar]
- Contaminants of emerging concern (CECs): Occurrence and fate in aquatic ecosystems. Int J Environ Res Public Health. 2021;18:13401.
- [CrossRef] [PubMed] [Google Scholar]
- Lindane (Gamma-hexachlorocyclohexane) exposure impairs [Ca2+]i-mediated vascular reactivity. FASEB J. 2018;S1:631.1.lb628.
- [CrossRef] [Google Scholar]
- Environmental chemicals disrupted lipid/fatty acid contents of rat organs and reversed by treatment with citrus lime nano-particle. FASEB J. 2020;34:1.
- [CrossRef] [Google Scholar]
- Toxicity of environmental contaminants. Biomed Res Int. 2015;2015:702439.
- [CrossRef] [PubMed] [Google Scholar]
- Resveratrol attenuates malathion induced damage in some reproductive parameters by decreasing oxidative stress and lipid peroxidation in male rats. J Family Reprod Health. 2019;13:70-9.
- [CrossRef] [PubMed] [Google Scholar]
- Hepatoprotective effect of Aloe vera against cartap-and malathion-induced toxicity in Wistar rats. J Cell Physiol. 2019;234:18329-43.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-inflammatory and antioxidative potential of Aloe vera on the cartap and malathion mediated toxicity in wistar rats. Int J Environ Res Public Health. 2020;17:5177.
- [CrossRef] [PubMed] [Google Scholar]
- Can antioxidants be beneficial in the treatment of lead poisoning? Free Radic Biol Med. 2000;29:927-45.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative study on in vitro inhibitory effects of heavy metals on rabbit drug-metabolizing enzymes. J Health Sci. 2001;47:14-20.
- [CrossRef] [Google Scholar]
- Clofibrate, a peroxisome proliferator-activated receptor-alpha (PPARα) agonist and its molecular mechanisms of action against sodium fluoride-induced toxicity. Biol Trace Elem Res. 2022;200:1220-36.
- [CrossRef] [PubMed] [Google Scholar]
- Carbofuran modulating functions of acetylcholinesterase from rat brain in vitro. Adv Biol. 2016;2016:3760967.
- [CrossRef] [Google Scholar]
- Toxic effects of organophosphate pesticide monocrotophos in aquatic organisms: A review of challenges, regulations and future perspectives. Environ Res. 2023;244:117947.
- [CrossRef] [PubMed] [Google Scholar]
- Oxidative stress, free radicals and antioxidants: potential crosstalk in the pathophysiology of human diseases. Front Chem. 2023;11:1158198.
- [CrossRef] [PubMed] [Google Scholar]
- Prenatal and childhood exposure to pesticides and neurobehavioral development: Review of epidemiological studies. Int J Occup Med Environ Health. 2008;21:121-32.
- [CrossRef] [PubMed] [Google Scholar]
- Total thiols: Biomedical importance and their alteration in various disorders. Online J Health Allied Sci. 2009;8:1-9.
- [Google Scholar]
- Superoxide dismutase: Dual roles in controlling ROS damage and regulating ROS signaling. J Cell Biol. 2018;217:1915-28.
- [CrossRef] [PubMed] [Google Scholar]
- Single and joint toxicity assessment of acetamiprid and thiamethoxam neonicotinoids pesticides on biochemical indices and antioxidant enzyme activities of a freshwater fish Catla. Comp Biochem Physiol Part C Toxicol Pharmacol. 2022;257:109336.
- [CrossRef] [PubMed] [Google Scholar]
- ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24:R453-62.
- [CrossRef] [PubMed] [Google Scholar]
- Harmful and beneficial role of ROS. Oxid Med Cell Longev. 2018;2018:5943635.
- [CrossRef] [PubMed] [Google Scholar]
- Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol Biochem. 2017;44:532-53.
- [CrossRef] [PubMed] [Google Scholar]
- Measurement and clinical significance of biomarkers of oxidative stress in humans. Oxid Med Cell Longev. 2017;2017:6501046.
- [CrossRef] [PubMed] [Google Scholar]
- Oxidative stress, aging, and diseases. Clin Interv Aging. 2018;13:757-72.
- [CrossRef] [PubMed] [Google Scholar]
- Clofibrate, a PPAR-α agonist, abrogates sodium fluoride-induced neuroinflammation, oxidative stress, and motor incoordination via modulation of GFAP/Iba-1/anti-calbindin signaling pathways. Environ Toxicol. 2020;35:242-53.
- [CrossRef] [PubMed] [Google Scholar]
- Luteolin attenuates glycerol-induced acute renal failure and cardiac complications through modulation of kim-1/NF-κB/Nrf2 signaling pathways. J Diet. ;18(Suppl 2021):543-65.
- [CrossRef] [PubMed] [Google Scholar]
- Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645-50.
- [CrossRef] [PubMed] [Google Scholar]
- Regulation of cytochrome P450 (CYP) genes by nuclear receptors. Biochem J. 2000;347:321-37.
- [CrossRef] [PubMed] [Google Scholar]
- Differential effects of 15-deoxy-Δ12, 14-prostaglandin J2 and a peroxisome proliferator-activated receptor γ agonist on macrophage activation. J Leukoc Biol. 2001;69:631-8.
- [CrossRef] [PubMed] [Google Scholar]
- Crosstalk between xenobiotic detoxication and other signalling pathways: Clinical and toxicological consequences. Xenobiotica. 2004;34:633-64.
- [CrossRef] [PubMed] [Google Scholar]
- Dysregulation of the peroxisome proliferator-activated receptor target genes by XPD mutations. Mole Cell Biol. 2005;25:6065-76.
- [CrossRef] [PubMed] [Google Scholar]
- Regulation of hepatic transporters by xenobiotic receptors. Curr Drug Metab. 2005;6:309-28.
- [CrossRef] [PubMed] [Google Scholar]
- Mode of action framework analysis for receptor-mediated toxicity: The peroxisome proliferator-activated receptor alpha (PPARα) as a case study. Crit Rev Toxicol. 2014;44:1-49.
- [CrossRef] [PubMed] [Google Scholar]
- PPARalpha agonists inhibit nitric oxide production by enhancing iNOS degradation in LPS-treated macrophages. Br J Pharmacol. 2007;152:1081-91.
- [CrossRef] [PubMed] [Google Scholar]
- Peroxisomes and disease-an overview. Int J Biomed Sci IJBS. 2006;2:308.
- [CrossRef] [PubMed] [Google Scholar]
- In vitro screening of 200 pesticides for agonistic activity via mouse peroxisome proliferator-activated receptor (PPARalpha and PPARgamma and quantitative analysis of in vivo induction pathway. Toxicol Appl Pharmacol. 2006;217:235-44.
- [CrossRef] [PubMed] [Google Scholar]
- Regulation of inflammation by PPARs: A future approach to treat lung inflammatory diseases? Fundam Clin Pharmacol. 2006;20:429-47.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation, and fibrosis in non-alcoholic fatty liver disease. J Hepatol. 2015;62:720-33.
- [CrossRef] [PubMed] [Google Scholar]
- The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409-35.
- [CrossRef] [PubMed] [Google Scholar]
- Antioxidant stress and anti-inflammation of PPAPa on warm hepatic ischemia-reperfusion injury. PPAR Res. 2012;2012:738785.
- [CrossRef] [PubMed] [Google Scholar]
- Role of peroxisome proliferators-activated receptors in the pathogenesis and treatment of nonalcoholic fatty liver disease. World J Gastroenterol. 2008;14:22.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of persistent toxic substances in the environment of Egypt. Environ Int. 2004;30:309-22.
- [CrossRef] [PubMed] [Google Scholar]
- Drinking water standards and health advisories United States: U.S. Environment Protection Agency Office of Water; 2000.
- [Google Scholar]
- Regulation of cerebrovascular endothelial peroxisome proliferator activator receptor alpha expression and nitric oxide production by clofibrate. Bratisl Lek Listy. 2010;111:258-64.
- [Google Scholar]
- Peroxisome proliferator-activated receptor alpha activation-mediated regulation of endothelin-1 production via nitric oxide and protein kinase C signaling pathways in piglet cerebral microvascular endothelial cell culture. J Pharmacol Exp Ther. 2007;320:774-81.
- [CrossRef] [PubMed] [Google Scholar]
- The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170-5.
- [CrossRef] [Google Scholar]
- Electrochemical reduction of Ellman's reagent: A novel selective detection protocol for thiol compounds. Electroanalysis. 2007;19:2437-43.
- [CrossRef] [Google Scholar]
- Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130-9.
- [CrossRef] [PubMed] [Google Scholar]
- Nitrite and nitrate measurement by Griess reagent in human plasma: Evaluation of interferences and standardization. Meth Enzymol. 2008;440:361-80.
- [CrossRef] [PubMed] [Google Scholar]
- Inducible nitric oxide synthase and inflammatory diseases. Mol Med. 2000;6:347-73.
- [CrossRef] [PubMed] [Google Scholar]
- The role of oxidative stress and antioxidants in liver diseases. Int J Mol Sci. 2015;16:26087-124.
- [CrossRef] [PubMed] [Google Scholar]
- Stress and combined exposure to low doses of pyridostigmine bromide, DEET, and permethrin produce neurochemical and neuropathological alterations in cerebral cortex, hippocampus, and cerebellum. J Toxicol Environ Health A. 2004;67:163-92.
- [CrossRef] [PubMed] [Google Scholar]
- Co-exposure to pyridostigmine bromide, DEET, and/or permethrin causes sensorimotor deficit and alterations in brain acetylcholinesterase activity. Pharmacol Biochem Behav. 2004;77:253-62.
- [CrossRef] [PubMed] [Google Scholar]
- Testicular germ-cell apoptosis in stressed rats following combined exposure to pyridostigmine bromide, N,N-diethyl m-toluamide (DEET), and permethrin. J Toxicol Environ Health A. 2003;66:57-73.
- [CrossRef] [PubMed] [Google Scholar]
- Neurological deficits induced by malathion, DEET, and permethrin, alone or in combination in adult rats. J Toxicol Environ Health A. 2004;67:331-56.
- [CrossRef] [PubMed] [Google Scholar]
- Endocrine disrupting chemicals in fish: Developing exposure indicators and predictive models of effects based on mechanism of action. Aquat Toxicol. 2009;92:168-78.
- [CrossRef] [PubMed] [Google Scholar]
- Angiotensin-(1-7) treatment blocks lipopolysaccharide-induced organ damage, platelet dysfunction, and IL-6 and nitric oxide production in rats. Sci Rep. 2021;11:610.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular mechanisms underlying chemical liver injury. Expert Rev Mol Med. 2012;14:e4.
- [CrossRef] [PubMed] [Google Scholar]
- The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr J. 2015;15:71.
- [CrossRef] [PubMed] [Google Scholar]
- Malathion, an organophosphate insecticide, provokes metabolic, histopathologic, and molecular disorders in liver and kidney in prepubertal male mice. Toxicol Rep. 2018;5:189-95.
- [CrossRef] [PubMed] [Google Scholar]
- Thiol redox homeostasis in neurodegenerative disease. Redox Biol. 2015;5:186-94.
- [CrossRef] [PubMed] [Google Scholar]
- Nitric oxide synthase regulation and diversity: Implications in Parkinson's disease. Nitric Oxide. 2006;15:280-94.
- [CrossRef] [PubMed] [Google Scholar]
- Redefining oxidative stress. Antioxid Redox Signal. 2006;8:1865-79.
- [CrossRef] [PubMed] [Google Scholar]
- Implications of plasma thiol redox in disease. Clin Sci (Lond). 2018;132:1257-80.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical relevance of biomarkers of oxidative stress. Antioxid Redox Signal. 2015;23:1144-70.
- [CrossRef] [PubMed] [Google Scholar]
- Role of thiols in oxidative stress. Curr Opin Toxicol. 2018;7:133-9.
- [CrossRef] [PubMed] [Google Scholar]
- Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol Cell Biol. 1995;15:3012-22.
- [CrossRef] [PubMed] [Google Scholar]
- Peroxisome proliferator-activated receptor alpha mediates the effects of high-fat diet on hepatic gene expression. Endocrinology. 2006;147:1508-16.
- [CrossRef] [PubMed] [Google Scholar]
- Role of ROS and RNS sources in physiological and pathological conditions. Oxid Med Cell Longev. 2016;2016:1245049.
- [CrossRef] [PubMed] [Google Scholar]
- Differential modulation of bradykinin-induced relaxation of endothelin-1 and phenylephrine contractions of rat aorta by antioxidants 1. Acta Pharmacol Sin. 2007;28:1566-72.
- [CrossRef] [PubMed] [Google Scholar]
- Inhibition of IFN-γ-mediated inducible nitric oxide synthase induction by the peroxisome proliferator-activated receptor γ agonist, 15-deoxy-Δ12, 14-prostaglandin J2, involves inhibition of the upstream Janus kinase/STAT1 signaling pathway. J Immunol. 2003;171:979-88.
- [CrossRef] [PubMed] [Google Scholar]
- Peroxisome-proliferator-activated receptors regulate redox signaling in the cardiovascular system. World J Cardiol. 2013;5:164-74.
- [CrossRef] [PubMed] [Google Scholar]
- P450 gene induction by structurally diverse xenochemicals: Central role of nuclear receptors CAR, PXR, and PPAR. Arch Biochem Biophys. 1999;369:11-23.
- [CrossRef] [PubMed] [Google Scholar]
- Acute toxication of deltamethrin results in activation of iNOS, 8-OHdG and up-regulation of caspase 3, iNOS gene expression in common carp (Cyprinus carpio L.) Aquat Toxicol. 2017;187:90-9.
- [CrossRef] [PubMed] [Google Scholar]
- Nitric oxide In: Encyclopedia of gastroenterology. United States: Academic Press; 2004. p. :731-2.
- [CrossRef] [Google Scholar]
- INOS-mediated nitric oxide production and its regulation. Life Sci. 2004;75:639-53.
- [CrossRef] [PubMed] [Google Scholar]
- The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature. 1998;391:79-82.
- [CrossRef] [PubMed] [Google Scholar]
- PPARs and molecular mechanisms of transrepression. Biochim Biophys Acta. 2007;1771:926-35.
- [CrossRef] [PubMed] [Google Scholar]
- Regulation of murine macrophage proinflammatory and anti-inflammatory cytokines by ligands for peroxisome proliferator-activated receptor-γ: Counter-regulatory activity by IFN-γ. J Leukoc Biol. 2002;71:677-85.
- [CrossRef] [PubMed] [Google Scholar]
- iNOS as a metabolic enzyme under stress conditions. Free Radic Biol Med. 2020;146:16-35.
- [CrossRef] [PubMed] [Google Scholar]
- Inhibition of IκB kinase and I?B phosphorylation by 15-deoxy-Δ12, 14-prostaglandin J2 in activated murine macrophages. Mol Cell Biol. 2000;20:1692-8.
- [CrossRef] [PubMed] [Google Scholar]
- Hepatoprotective effects of vitamin E/selenium against malathion-induced injuries on the antioxidant status and apoptosis-related gene expression in rats. J Toxicol Sci. 2011;36:285-96.
- [CrossRef] [PubMed] [Google Scholar]
- Link between free radicals and protein kinase C in glucose-induced alteration of vascular dilation. Life Sci. 2004;75:2921-32.
- [CrossRef] [PubMed] [Google Scholar]







