Translate this page into:
In pursuit of incorporation of markers of oxidative stress in traditional biochemical panels in clinical Chemistry: A risk assessment step in diagnosis and biotherapy

-
Received: ,
Accepted: ,
How to cite this article: Anetor JI, Uche CZ, Anetor GO. In pursuit of incorporation of markers of oxidative stress in traditional biochemical panels in clinical Chemistry: A risk assessment step in diagnosis and biotherapy. Am J Biopharm Pharm Sci 2022;2:1.
Abstract
Chemical pathology (clinical chemistry/biochemistry) is the branch of laboratory medicine concerned with the detection of alterations in the chemical constituents and biochemical mechanisms, which ensure health, culminating in disease. The disease itself is a pattern of response to some insult or injury resulting in a disturbed function or structure. It is often difficult to ascertain precisely the point of transition from health to a disease state. Pathological changes, including metabolic and molecular perturbations, with the potential to progress to clinical disease, are also present in healthy populations, noteworthy are the reactive oxygen species such as hydroxyl radicals with the propensity to cause oxidative DNA damage. Biochemical profiles or panels such as liver function tests, renal function tests, bone profile, lipid profile, acid-base, and critical care have served as biomarkers employed in indicating the presence of or measuring the progress of the disease, as well as the effect of treatment. Oxidative stress, an imbalance between bio-available antioxidants and reactive species, is now widely recognized as accompanying most pathological states. Hence, the exclusion of antioxidant components in biochemical profiles appears a grave oversight. Basic components of the antioxidant system, glutathione (GSH), zinc, uric acid, ascorbic acid, and α-tocopherol, may be selected for incorporation. GSH is particularly important; as a scavenger for damaging oxidative intermediates in cells, it promises to be a good predictor of disease progression and prognosis. Including the antioxidant component into traditional profiles may aid physicians in more confidently ruling out disease, enabling further investigations, and/or reassuring patients. It is proposed that redefining the traditional profiles in chemical pathology by incorporating the indexes of the antioxidant system promises considerable improvement in the risk assessment process, in disease detection and recognition of the threshold of clinical concern in disease management and biotherapy.
Keywords
Antioxidant hypothesis
Biochemical profiles
Chemical pathology
Disease detection
and biotherapy
Glutathione
Oxidative stress markers
Highlights
Panels or profiles have been employed in chemical pathology for a long time because it is often more informative to measure several biochemical variables together
The use of biochemical profiles may be applied more appropriately in investigations after a hypothesis has been formulated for an individual patient based on the clinical picture, and sensitive tests are chosen to confirm or exclude the diagnosis as quickly as possible
The health of the individual is at least in part maintained by a potent antioxidant system, particularly in preventing, delaying, or eliminating several chronic pathologic states
Physiologically, antioxidants prevent damage to cellular components arising from chemical reactions involving free radicals. Deficits of antioxidants to perform this role lead to excess free radicals and a state of oxidative stress. A role for oxidative stress has been postulated and demonstrated in many pathological states, such as atherosclerosis, diabetes mellitus, cancer, inflammation, and aging
Determination of total antioxidant status along with the biochemical profiles is potentially useful. It has the potential to play a beneficial role in the identification of an organ or system dysfunction in patients with prepathologic (occult pathologic) states. Alternatively, key components of the antioxidant system such as glutathione (GSH), zinc, uric acid, ascorbic acid, and α-tocopherol may be selected for incorporation into current standard chemical pathology profiles. GSH is particularly important; it acts as a scavenger for damaging toxic free radicals in the cell. Very importantly, incorporating GSH promises to be a good predictor of disease progression, prognosis, severity, and response to treatment
Evolving evidence suggests that the time has come for the scientific community to put the fruits of decades of research in the field of antioxidant and associated oxidative stress into routine chemical pathology practice. This is already being done in therapeutics. Many people today take supplements for prophylaxis and adjunct therapy based at least in part on the antioxidant hypothesis. It is suggested that this should be extended to clinical chemistry; an important component of the disease diagnosis and therapy system.
INTRODUCTION
Chemical pathology, also variously known as clinical biochemistry, clinical chemistry, and at times medical biochemistry, is the study of the alterations in the chemical constitution and biochemical mechanisms of the body as a result of the disease. Chemical pathology is concerned with the biochemical basis of disease processes, their laboratory investigation, diagnosis, treatment, and prevention. The main uses of chemical pathology as with other branches of pathology are shown in [Table 1]. Panels or profiles have been employed in chemical pathology for a long time. This is because it is often more informative to measure several biochemical variables at the same time.[1] The commonly measured profiles are shown in [Table 2]. Biochemical profiles may not often be diagnostic in themselves. They, however, may point to a diseased organ system or a deranged metabolic process. The use of biochemical profiles may be applied to be more appropriately investigated after a hypothesis has been formulated for an individual patient based on the clinical picture, and sensitive tests are chosen to attempt to exclude the diagnosis as quickly as possible or on the contrary, confirm a diagnosis.[2]
| S. No. | Main uses | Examples |
|---|---|---|
| 1. | Assistance in making a diagnosis | Differential diagnosis of tiredness – may be chronic kidney disease |
| 2. | Confirmation of a diagnosis | Determination of fasting plasma glucose in a case of polydipsia, polyuria, and polyphagia |
| 3. | Assessment of clinical progress | Periodic determination of transaminases level in acute hepatitis |
| 4. | Assistance with prognosis | Measurement of microalbumin in DM |
| 5. | Assistance with control of treatment | Periodic measurement of glucose or HbA1c in Type 1 DM on treatment |
HbA1c: Hemoglobin A1c, DM: Diabetes mellitus
| S. No. | Traditional profiles in clinical chemistry | Parameters |
|---|---|---|
| 1. | Liver function tests | Total protein, albumin, bilirubin, ALP, AST, ALT, LD, GGT, and urea |
| 2. | Renal profile | Total protein, albumin, urea, creatinine, and phosphorus. |
| 3. | Bone profile | Calcium, phosphorus, alkaline phosphatase, and albumin |
| 4. | Hematopoietic system | LD, uric acid, and bilirubin. |
| 5. | Lipid profile | Total cholesterol, triglycerides, HDL, and LDL |
| 6. | Cardiovascular disease panel | CK AST, troponins, and myoglobin |
| 7. | Thyroid function panel | T4, T3, TSH, FT4 |
| 8. | Malnutrition panel | Albumin, RBP, Transthyrectin, Ferritin, Hb, Transferrin |
| 9. | Pregnancy monitoring panel | Fe, Folate, hematocrit, urinary albumin, plasma glucose, and urate |
| 10. | Toxicity panel | Toxicant of interest in blood or urine or its metabolites |
ALP: Alkaline phosphatase, ALT: Alanine aminotransaminase, AST: Aspartate aminotransferase, GGT: Gamma-glutamyl transferase, LD: Lactate dehydrogenase, CK: Creatine kinase, LDL: Low-density lipoprotein, HDL: High-density lipoprotein, TSH: Thyroid-stimulating hormone, PAB: Pre-albumin, RBP: Retinol-binding protein, Fe: Iron, T3: Triiodothyronine, T4: Thyroxine, FT3: Free triiodothyronine, Hb: Hemoglobin
THE ANTIOXIDANT SYSTEM IN HEALTH AND DISEASE: CENTRAL ROLE IN CLINICAL CHEMISTRY
The health of the individual at least in part is maintained by a potent antioxidant system. Antioxidants may be referred to as any substance that when present in low concentrations compared to oxidizable substrate significantly delay or inhibit the oxidation of that substrate.[3,4] From the physiological perspective, antioxidants prevent damage to cellular components arising from chemical reactions involving free radicals. When there is an insufficient antioxidant to perform this role, leading to excess free radicals, a state of oxidative stress is said to exist. A role for oxidative stress has been postulated and demonstrated in many pathological states, ranging from atherosclerosis to cancer, inflammation, and aging.[5,6] These diseases are common conditions investigated in chemical pathology or clinical chemistry.
The disease itself is a pattern of response to some insult or injury resulting in disturbed function or structure. It is difficult to pinpoint precisely the point of transition from a healthy to a disease state. Pathological changes including metabolic and molecular perturbations with the potential to cause disease are present in healthy populations. Reactive oxygen species (ROS) such as hydroxyl radicals can damage DNA. If nucleotide bases are altered or destroyed, mutation can ensue and if some genes are mutated, cancer or other degenerative diseases can set in.[7] Biochemical profiles or panels such as liver function tests (LFTs), renal and bone profiles, and lipid profiles have been employed as biomarkers; the biochemical or genetic features of which can be harnessed to measure the progress of disease or the effect of treatment. The antioxidant hypothesis which posits that oxidative damage by ROS can have long-term implications, at least in part fulfills the definition of a biochemical panel. Antioxidants refer to substances capable of protecting biomolecules from the damaging effects of reactive species.
Oxidative stress is the imbalance between bioavailable antioxidants and reactive species, which is now widely recognized as an accompanying component of almost all pathological states.[5] It is, therefore, surprising that while the practice of using biochemical panels has been traditionally employed to identify the onset or presence of disease, the role of the antioxidant system has been relatively neglected in routine clinical chemistry practice. For instance, the total antioxidant status determination alongside other biochemical profiles promises to be very useful in disease diagnosis and prognosis. It has the potential to play a beneficial role in the identification of organ or system dysfunction(s) in patients with pre-pathologic (occult pathologic) state(s). Alternatively, key components of the antioxidant system(s) such as GSH, zinc, uric acid, ascorbic acid, and α-tocopherol may be selected for incorporation in the current standard chemical pathology profile. GSH particularly is important as a scavenger for damaging oxidative metabolites in the cells and promises to be a good predictor of disease progression, prognosis, and severity.[8] The antioxidant status may indeed offer, at least in part some degree of explanation for the variable presentations of patients with the same disease, comorbidities, etc., aside from genetics.
Antioxidants prevent damage to DNA, lipids, and proteins including antibodies, by efficiently scavenging free radicals with far-reaching biological and clinical implications, as well as immunocompetence and quicker recovery from disease. Many biochemical profiles even when adjudged normal may not necessarily exclude disease. Consequently, incorporating the antioxidant component into traditional biochemical profiles may help the physician to more confidently rule out disease and a rational indication for further investigations and/or reassurance of the patient. It is thus considered that redefining traditional profiles in clinical chemistry by incorporating aspects of the antioxidant system may substantially improve the risk assessment process in disease detection and disease management. The firm belief of many investigators in this field is that the time has come for the scientific community to put the fruits of decades of research in the antioxidant hypothesis into the routine chemical pathology practice.[5,9] This is almost routinely being done in therapeutics. Many people today take supplements inadvertently, based in part on the antioxidant hypothesis. It is, therefore, considered an important omission in laboratory medicine service. As indicated in [Figure 1], antioxidants are central to the emergence of many pathologic states.[4,5]

- The progression of weakened antioxidant status to disease. (Source: Anetor, 2009).[10]
Few laboratory tests are pathognomonic, that is, specific for one disease to the exclusion of other diagnoses. As the diagnostic method remains unchanged, from a general impression based on the patient’s appearance, history, and physical signs of localization of the focus of inquiry to a particular organ or body system, there is an increasing tendency to group laboratory investigations into operational sets or panels (profiles). The advent of multichannel and multitest automated analyzers has promoted the use of these profiles in chemical pathology.[11] If employed responsibly and intelligently, the biochemical profiles can quicken the diagnostic process by the more rapid exclusion of alternatives. A common set of tests for the diagnosis of liver disease may include such investigations as serum total and conjugated bilirubin, the transaminases, alanine aminotransaminase, and aspartate aminotransferase, alkaline phosphatase, as well as serum proteins including electrophoretic fractionation; gamma-glutamyl transferase (GGT) (GGT is indirectly already involved in the redox system, due to its role in the transfer of glutamyl moiety in GSH synthesis), and urine for bile pigments. A typical profile for kidney disease may include urea, creatinine, creatinine clearance, urinalysis, incorporating albumin/creatinine ratio, serum proteins, and urine and serum osmolality. These can be improved by incorporating aspects of the antioxidant system such as modified albumin,[12] as shall be evident shortly and as earlier investigators have observed directly or indirectly.[5]
ANTIOXIDANTS
Antioxidants are the organism’s key protective system against the inevitable constant generation or exposure to free radicals which may be either endogenous or exogenous. Many diseases and exposures lead to the generation of free radicals, which are ameliorated by antioxidants. One of the body’s most important antioxidants is GSH, a tripeptide composed of glycine, cysteine, and glutamic acid. Many disorders and exposures to xenobiotics deplete GSH from the liver, kidneys, heart, and many other tissues.[13] Adding the antioxidant components to current panels may provide a better assessment paradigm for individuals with distinct pathologic states including infections that are a current challenge to the global community. Another common antioxidant, often, overlooked is Vitamin C, an aqueous antioxidant vitamin required for optimal activity of several enzyme systems involved in many biosynthetic pathways in the human body.[14]
Oxidative biomarker investigations employing ascorbic acid have shown that the vitamin protects against in vivo oxidation of many macro biomolecules, such as lipids, and DNA in humans, particularly at-risk groups. It also has antioxidant, anti-inflammatory, and immunomodulatory properties.[14] Several epidemiological studies suggest strongly that antioxidants like Vitamin C lower the incidence and associated mortality of some of the most prevalent pathologic states such as cancer and cardiovascular disease, infections, and sepsis.[14] Indeed, it has been suggested that it may be worth measuring Vitamin C levels in patients and treating them with intravenous administration within intensive care units and oral administration in hospitalized persons with COVID-19.[14]
THE ANTIOXIDANT HYPOTHESIS
The antioxidant hypothesis is essentially based on the concept that increased free radical burden may lead to an imbalance with available antioxidants, culminating in oxidative stress which can be counteracted by the bioavailability of adequate antioxidants.[15] At present, the hypotheses despite some expression of dissension, largely arising from analytical inconsistencies, favor the idea that lowering oxidative stress has some clinical benefits. Unequivocally, a weakened antioxidant defense leads to oxidative stress which, in turn, leads to oxidative damage(s) and ultimately in diseases such as heart disease, diabetes, or at least its complications and cancer mentioning only a few [Figure 1]. The antioxidant hypothesis implies that enhanced antioxidant status is associated with decreased disease risk. Hence, oxidative stress may be considered a key underlying factor in health and disease.[16] More and more evidence continues to indicate that a proper balance between oxidants and antioxidants is involved in maintaining health and longevity and that altering this balance in favor of oxidants may result in pathological responses causing functional disorders and disease or complications and/or poor prognosis in established pathologic states.[17] The potential for predicting normal or near-normal health and disease prevention may necessitate including indicators of antioxidant status in traditional profiles in chemical pathology. It is almost no longer in doubt that free radicals are involved in human diseases, their severity, prognosis, and management.[5,9] This is supported by the abundant evidence from the current literature in basic medical science and clinical medicine.[17-19]
Persistent oxidative stress due to ROS, particularly the superoxide anion; the primary type of ROS generated through various cellular metabolic pathways and exposure to ionizing radiation, causes direct damage to the DNA and other macromolecules resulting in DNA strand breaks, mutations, cytotoxicity, genomic instability, etc., which are the hallmark of most diseases [Figure 2].
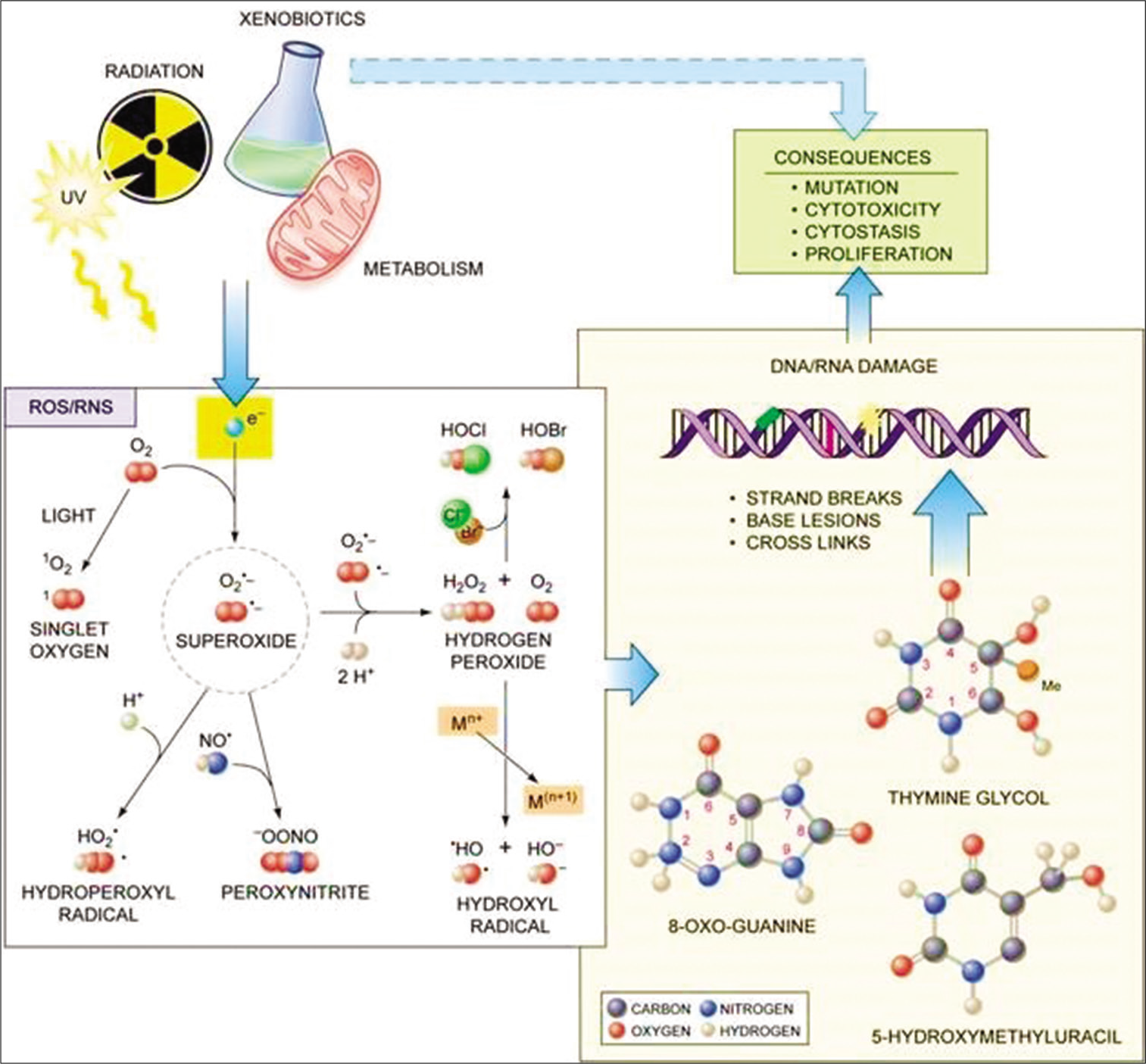
- Scheme showing key players in oxidative stress and their possible role in risk assessment in clinical chemistry. Courtesy: Professor Marcus Cooke, Formerly of Oxidative Stress Group, Florida International University, Miami Florida.
Convincingly, free radicals have been demonstrated to be involved in numerous diseases. Halliwell and Gutteridge[17] pointed out that a wide range of disorders indicated a raised burden of free radicals or reactive species and that it is not an unusual phenomenon. Other studies further pointed out that it may be associated with tissue injury in most if not all human diseases.[4,5] The reason for this is not farfetched; largely mechanisms of the biology of diseases such as liberation of transition–metal ions, some specific proteins, mobilization of phagocytes, and damage to key organelles such as the mitochondria in turn leading to increased leakage of the constituents of the electron transport chain. Other changes may include loss of antioxidants such as ascorbate, plasma thiols (albumin-SH group), and importantly GSH.[20] A secondary mechanism may be compromised antioxidant nutrient levels in ill individuals. This appears a shred of compelling evidence to include antioxidant indices in the panels of profiles for the assessment of disease states as a form of risk assessment and predictive process, all rolled into the investigative process commonly undertaken in most chemical pathology or clinical chemistry laboratories.
This derives directly from the understanding that illness is inevitably associated with abnormal biochemistry and an alteration in the metabolism of nutrients and their metabolites. Thus, by correcting fundamental biochemical derangements using nutritional means one can prevent certain diseases or modulate the progress of a disease for a better prognosis. This may be better achieved if there are markers of the antioxidant status incorporated into the investigative process or panels. This approach appears consistent with the observation of Gey and Brubacher[21] who reported risk thresholds for antioxidant blood levels in relationship to cancer and heart disease prevention. The investigators consequently proposed biological monitoring of antioxidants as a new approach to cancer prevention. Essentially, these researchers examined the relationship between plasma levels of antioxidant vitamins in relation to cancer and coronary heart disease. They proposed a novel approach to detecting antioxidant vitamin deficiencies which may predict the future risk of these common diseases in the population. From their data, desirable plasma levels and the corresponding daily consumption of the most important antioxidants or anti-cancer vitamins provided evidence of plasma levels of essential antioxidants associated with reduced risk of major health hazards and consequently recommended prudent intake to achieve potentially protective plasma levels in the population they studied. As a clinical deduction from this study, Tolonen[22] proposed that the desirable selenium level in whole blood is shown to correlate with reduced lipid peroxidation in humans as 200–350 μmol/L. The previous studies have established that increased lipid peroxidation has the potential of increasing the future risk of cancer and cardiovascular diseases.
THE LIVER
The liver is the central organ for detoxification of xenobiotics and one of the organs in the body with the most intense metabolic activities. The liver is also the major organ involved in pharmacokinetic (PK) and pharmacodynamics activities. Essentially, this involves the effects of drugs on the body and or the response of the body respectively, as well as the biochemical and physiological consequences of such interactions. These processes may lead to the generation of ROS that may need scavenging, which if inappropriately handled may lead to oxidative stress. The liver is also the major storage site of GSH, a very important antioxidant, hence may be considered an exporter of GSH to other sites of the body. Interestingly, the concentration of GSH is highest in the functional cells of the liver; the hepatocytes and reaches a concentration of about 10 mm in the healthy liver,[23] suggesting that GSH plays a critical role in the functioning of the liver. Corollary, its assessment as in the traditional LFTs will be better assessed by incorporating aspects of the antioxidant system. The liver also plays an important role in nutrition. It is the storage site of Fe, Vitamin A, and several others which play various roles in the antioxidant system[24,25] through the Haber–Weiss reaction. GSH level is depleted in malnutrition or starvation.[26] Sherwood and Bomford,[27] though did not outrightly suggest incorporation of components of the antioxidant system in the assessment of liver function, indirectly alluded to the benefit of the antioxidant system in clinical conditions.
GSH AND LIVER FUNCTION
The liver is one of the most well-established organs commonly investigated with a battery of tests to obtain comprehensive information about its functional state. Notably, LFTs have been employed for a long time, while other vital functions of the liver such as GSH metabolism have remained largely neglected. GSH is a tripeptide antioxidant in the aqueous phase and an important cofactor for enzymatic antioxidants. This tripeptide plays an important role in the gamma-glutamyl circle. It is, particularly important in the liver, in that it is most concentrated in this organ (10 mM),[23] therefore, serving as a repository for GSH supplies to other tissues.
GSH is involved in the conversion of lipid-soluble substances to water-soluble/easily excretable forms through the kidney. It also plays an important role in PKs and serves as a systemic source of thiol (-SH) reducing power. Depletion of GSH leads to cell death, which has been observed in many pathologic states and has been suggested to be the ultimate determinant of susceptibility to oxidative states.[23] Another aqueous phase antioxidant, ascorbic acid as seen above,[14] has been reported to help in conserving GSH.[28] Thus, to fully realize the antioxidant potential of GSH, by incorporating GSH as a component of the traditional liver function profile, it will be appropriate to include ascorbic acid. A typical LFT profile may thus be as follows [Table 3]:
| S. No. | Type of test/application | Analytical protocol | References |
|---|---|---|---|
| 1. | Total bilirubin – excretory capacity | Diazo reaction | Michaelson et al. Paediatrics 1965;35:925 |
| 2. | Conjugated bilirubin – conjugating capacity | Diazo reaction | Michaelson et al. Paediatrics 1965;35:925 |
| 3. | Total protein – nonspecific | Spectrophotometry: Classical Biuret reaction | Lubran MM Ann Clin. Lab. Sci 1978l 8:106-110 |
| 4. | Albumin – synthetic ability | Spectrophotometry (dye binding; BCG/BCP) | Bartholomew R J and Delaney AM. Proc. Aust Assoc Clin Biochem 1966, 1:214 |
| 5. | Total globulin-immune response | Hopkins-Cole reaction | Goldenberg H, Drewes PA Clin Chem 1971;17:358 |
| 6. | Alkaline phosphatase – assess cholestasis | Continuous spectrophotometry | Bowers GN McCombs RB Clin Chem 1966;12:70-89 |
| 7. | Alanine aminotransferase – hepatocellular damage | Continuous monitoring method | Scandinavian Committee on Enzyme J Clin Lab Invest 1974; 33: 291 |
| 8. | Aspartate aminotransferase – hepatocellular damage | Continuous monitoring method | Scandinavian Committee on Enzyme J Clin Lab Invest 1974;33: |
| 9. | ϒ-glutamyl transpeptidase – cholestasis/intoxication | Continuous monitoring method | Scandinavian Committee on Enzyme J Clin Lab Invest 1976;37: |
| 10. | Glutathione – oxidative stress | Coupled spectrophotometry | Zheng X et al. Anal Methods 2015:12 |
| 11. | Ascorbate – antioxidant effect and oxidative stress | HPLC/Spectrophotometry 2, 6-dichlorophenplndophenol reaction | Robitaille L, Hoffer LJ. Nutr J 2016; 15: 40 |
HPLC: High-performance liquid chromatography, BCG: Bromocresol green, BCP: Bromocresol purple
ASCORBATE AND TOCOPHEROL RELATIONSHIP TO GSH AND DISEASE
It is remarkable that enzymes collectively referred to as GSH transhydrogenase use GSH as a cofactor to reconvert dehydroascorbate to ascorbate. As for ascorbate, GSH through its enormously reducing power, contributes to the recycling of other oxidized antioxidants, key among these is alpha-tocopherol (vitamin E). This probably explains the ability of GSH to conserve lipid-phase antioxidants including, the carotenoids. Meister and Larsson,[28,29] by employing buthionine sulfoximine (BSO) to inhibit GSH synthesis in animal models, demonstrated that GSH almost certainly promotes conservation of lipid phase antioxidants. This probably qualifies Vitamin E to be included in the above list [Table 3]. It should also be recalled that ascorbate plays an important role in reconverting tocopheryl radicals (oxidized) to the functional alpha-tocopherol.
Studies on the relationship between ascorbate and GSH have revealed that dietary ascorbate can protect against the tissue damage that characteristically accompanies GSH depletion.[29-32] This experiment was largely based on the role of L-gluconolactone oxidase which some animals lack including humans and thus cannot synthesize ascorbate. In animal models such as adult rats and mice that can synthesize adequate amounts of ascorbate, GSH depletion was not lethal. In contrast, in animals that cannot synthesize ascorbate such as guinea pigs, GSH depletion may culminate in death.[33] To provide further evidence of the role of ascorbate, supplementation of diet with ascorbate protected the experimental animals against GSH depletion. The converse also holds with GSH in animals deficient of GSH and given ascorbate.[34]
DIABETES MELLITUS (DM)
This metabolic disease is now recognized to be associated with considerable oxidative stress probably arising from the chronic hyperglycemia characteristic of DM. Oxidative stress may be particularly important in accelerated atherosclerosis and microvascular damage of the retina (retinopathy), kidney (nephropathy), and nerves (neuropathy).[35,36] The ultimate mechanism underlying this may be because, in hyperglycemia, the polyol pathway is enhanced while aldose reductase, one of the enzymes in the pathway, converts excess glucose to sorbitol using and consuming NADPH on which GSH reductase activity is dependent. By corollary, this through several steps leads to the accumulation of GSH disulfide, leading to a reduction of GSH, an important intracellular antioxidant, resulting in oxidative stress, now associated with DM and inversely correlated with DM complications,[37] but rarely assessed in the routine evaluation of the diabetic profile, which seems to remain traditionally pegged on fasting plasma/random glucose, hemoglobin A1c (HbA1C), creatinine, urea, total cholesterol, and high-density lipoprotein (HDL).
Although it is unsettled if oxidative stress causes diabetes,[38] it is, however, generally accepted that diabetes is associated with oxidative stress. Several markers of oxidative stress such as lipid peroxides and F2-IPs are reported to be raised in diabetic patients.[39] There is a lack of agreement if Vitamin E levels are reduced in diabetics. What appears to be fairly agreed on and is not surprising by most investigators is that two other key antioxidants ascorbate and GSH levels are reduced.[40,41] Glucose has also been reported to glycate CuZn-SOD in red blood cells and in the process lessening the activity of major antioxidants, which may at least in part explain the reduced SOD level observed by some investigators.[40] It has been demonstrated that CuZn-SOD and ceruloplasmin can dissociate post-glycation and release pro-oxidants such as copper ions.[42] Glycation products themselves are oxidizable by a number of reactive species, such as OH. and ONOO- giving rise to advanced glycation products (AGEs). The accumulation of these products in collagen can lower the connective tissue (blood vessel function abnormality) damage the basement membrane of the kidney. Although the implications of oxidative stress in diabetic pathology are incompletely understood, it is most probable that it contributes to the key complications of the disease such as the increased pace of atherosclerosis, diabetic nephropathy, neuropathy, and retinopathy.[17,41,43] Thus, this key metabolic disease and one of the frontline disorders traditionally investigated in chemical pathology will benefit from the inclusion of at least the antioxidants; Vitamin C, and GSH to the traditional profile of its investigation and management.
ATHEROSCLEROSIS/CARDIOVASCULAR DISEASE
The risk of atherosclerosis is often assessed by examining the lipid profile consisting of a total cholesterol, triglycerides, low-density lipoprotein cholesterol, HDL, and total cholesterol/HDL ratio. Atherosclerosis is associated with oxidative damage to the wall of the vessels. Oxidative stress is central to cardiovascular diseases.[44] Several risk factors are associated with cardiovascular diseases such as hypertension, hypercholesterolemia, DM, and habits such as cigarette smoking. In chemical pathology, risk and prognosis are often assessed with the traditional lipid profile indicated above. It is also known that CVD itself generates substantial ROS in the vascular wall. Superoxide, a key ROS and one of the products of vascular wall generation, may react with nitric oxide (NO) culminating in peroxynitrite (ONOO-) in the process of reducing NO production, which is beneficial in vascular physiology. A decrease in NO concentration is considered a key mechanism of cardiovascular disease. In addition, the existence of oxidative stress is thought to induce atherogenesis through various pathways among which are activation of transcription factors, which, in turn, stimulate the expression of pro-inflammatory factors and induction of peroxidation and protein oxidation products.[45] These studies at least in part are suggestive of a useful role of antioxidants and thus extremely rational to suggest that including antioxidant parameters may prove to be beneficial in the assessment risk of cardiovascular disease or its prognosis. Elevated levels of lipid peroxides, reduced GSH peroxidase levels, and related constituents of this antioxidant system have been reported in humans and animal models of atherosclerosis.[43] GSH can produce coronary vasodilation when added to isolated perfused rodent hearts, probably arising from its ability to regulate prostaglandin synthesis.[46]
PANCREATITIS
This disease is an inflammatory disorder that may be acute, acute recurrent, or chronic. It may arise from free radical damage.[47] These patients often suffer from concomitant antioxidant depletion.[23] This may not be surprising as the condition is often associated with impaired digestion of lipids due to lipase alteration resulting in the accumulation of lipids that may generate lipid peroxides. Studies have shown increased lipid peroxidation products in pancreatic juice, duodenal juice, and the bile of patients who suffer from pancreatitis. Some patients whose pancreatitis probably arose from alcohol-induced pancreatitis, with marked GSH reduction as reflected by increased oxidized GSH (GSSG), on treatment with a precursor of GSH, N-acetylcysteine (NAC), showed impressive improvement within a couple of days; indicating a role for antioxidants in this disorder. This was followed by a randomized clinical trial of NAC in patients suffering pancreatitis. These trials with NAC demonstrated a significantly improved clinical state in these patients in about 3 days.[48]
MALNUTRITION
Malnutrition is a common problem in which oxidative stress has been implicated and is global, though more prevalent in developing countries or countries with transition economies. While the affluent countries are worried about excess nutrition leading to obesity, the resource-poor countries are preoccupied with deficiency states, extremes of which may present as either marasmus or kwashiorkor. More worrisome and often poorly recognized is the so-called hidden hunger which largely comprises the micronutrients; the source of antioxidants. While the antioxidant hypothesis is important in both overnutrition and deficiency states, it needs to be given special emphasis. The report of Schofield and Ashworth[49] may be appropriate here. They recommended that all children should receive Vitamin A on presentation as well as 5 mg of folic acid. They further suggested that this could be followed by a multivitamin supplement daily and a mineral mix, in conjunction with refeeding formula or rehydration solutions. They gave these recommendations to correct subclinical, but important deficiencies in minerals such as potassium, phosphate, magnesium, and zinc. It is noteworthy that the antioxidant hypothesis was unwittingly upheld here. These nutrient supplements (mixture of vitamins and minerals), according to these investigators, contain antioxidants that are important in the repair of tissues injured by free radicals, again confirming the importance of the antioxidant system in this condition, alluding to associated oxidative stress. Hence, moderate-to-mild micronutrient deficiencies could lead to impaired DNA repair mechanisms cumulating in genomic instability and disease [Figure 3].
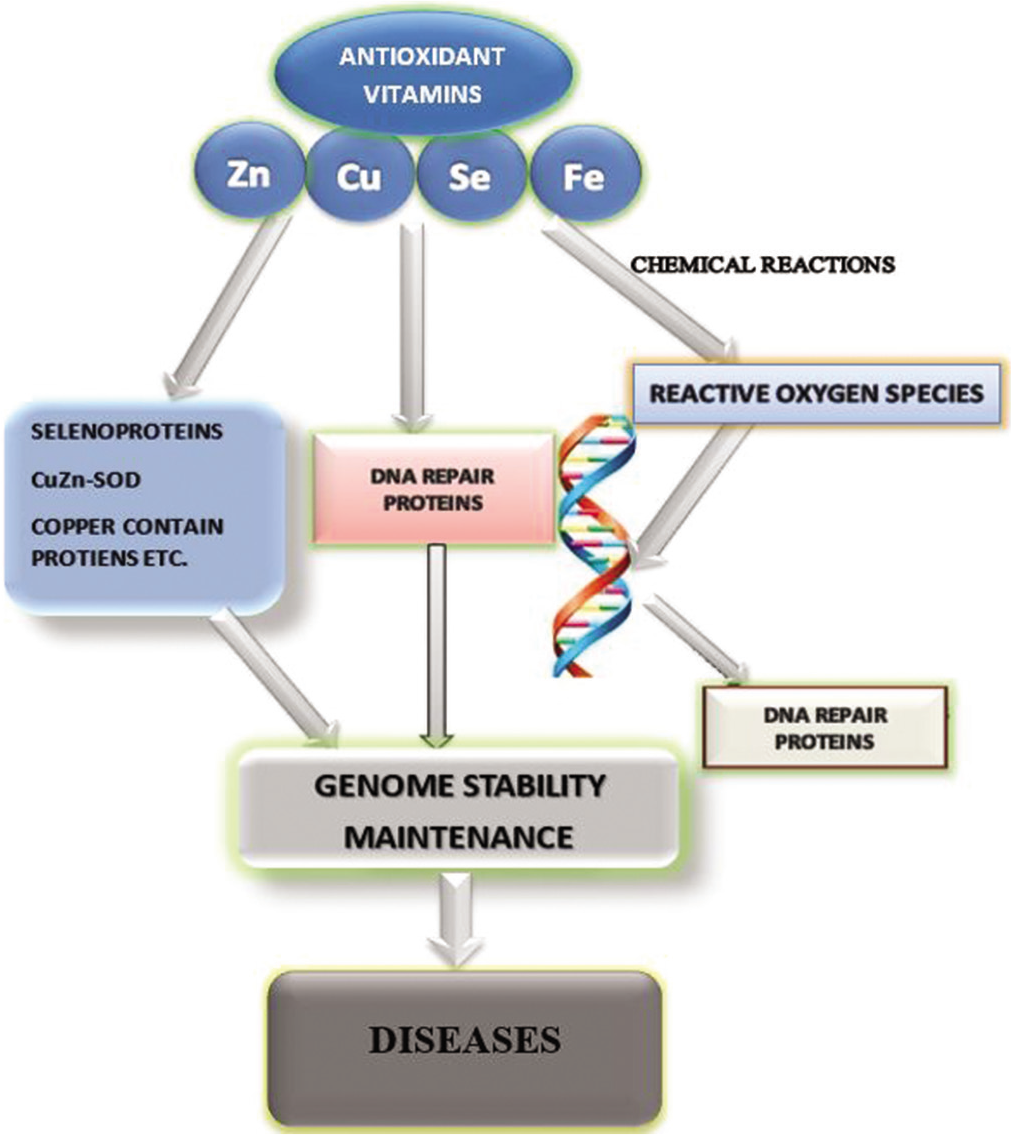
- Schematic diagram of nutritional elements and their roles in preventing oxidative stress. (Adapted and modified from Cheng, 2009).[51]
Iron (Fe), an important nutrient commonly used in the management of iron deficiency anemia, is also a pro-oxidant (free radical generator; Fenton reaction below shows the generation of the potent hydroxyl radical) and therefore often not recommended in the initial phase of refeeding in malnutrition.[49]
Schofield and Ashworth[49] have decried the continuous prescription of Fe supplements in 39% of centers in Africa. The management guidelines recommend transfusion for severe anemia and oral iron only after initial refeeding and these investigators believe that the inadvertent neglect of the antioxidant hypothesis may be the reason why mortality rates for severe malnutrition remain so high. Therefore, in addition to the already listed benefits of including oxidative stress markers in traditional panels in clinical chemistry, oxidative stress may suggest the risk of mutation in the somatic cell that may put individuals at the risk of cancer.[50] This knowledge may lead to early recognition of the risk of genomic and post-genomic alterations that may give rise to several pathological states. The incorporation of markers of oxidative stress may also point to the likelihood of chronic degenerative diseases, which share several well-recognized risk factors for the pathways of pathogenesis that may include oxidative stress, DNA damage, or inflammation.[50]
APPROACH IN DISEASE PREVENTION
The approach enunciated above may contribute to disease prevention commonly agreed to operate at three levels as follows, primary prevention, secondary prevention, and tertiary prevention. Primary prevention is considered to refer to preventing the occurrence of disease, while secondary prevention may refer to early detection and intervention, preferably before the condition becomes clinically manifest and has the potentials of reversing, arresting, or at least retarding the progress of the pathologic state.[53] The third phase of the preventive approach by including oxidative stress markers in common biochemical panels in clinical biochemistry is tertiary prevention, which implies minimizing the effect of disease by among others preventing the development of complications and early onset of deterioration.[53] This has been highlighted by previous reports from investigators in the field.[17,19,20] We had also previously reiterated this in our study on mixed chemical exposure, a ubiquitous problem in rapidly industrializing countries with associated rapid urbanization.[54] Prasad[55] indirectly, in supporting the goal of this report and using zinc, a well-known deficiency state which is more common in low- and medium-income countries noted that zinc is a second messenger of the immune system and that zinc is not only modulating cell-mediated immunity (CMI) but is also an antioxidant and anti-inflammatory agent.
This observation appears to suggest the need to include this essential micronutrient and antioxidant in existing biochemical panels in clinical chemistry that may aid the risk assessment of diseases.
The important knowledge that Zn is estimated to account for about 10% of the entire genome proteins and the fact that over 2000 zinc-dependent transcription factors play key roles in gene expression of various proteins,[56] strengthens the argument to revise the current panels. Notably, zinc deficiency had long been predicted to adversely affect several transcription factors with far-reaching clinical correlates. Some of the transcription factors that have been demonstrated to be grossly affected adversely as a consequence of Zn deficiency include; NFkB, AP-1, SP-1, A20, T-bet, and STAT-4,[34,57] all of which play crucial roles in metabolic homeostasis and health. [Figure 4] shows the many molecular and cellular events in which Zn plays important roles including oxidative stress [Figure 4]. A quick example of the predictive role of markers of oxidative stress may be the role of Zn in DM, a metabolic disease of great concern because of its global explosion recently.
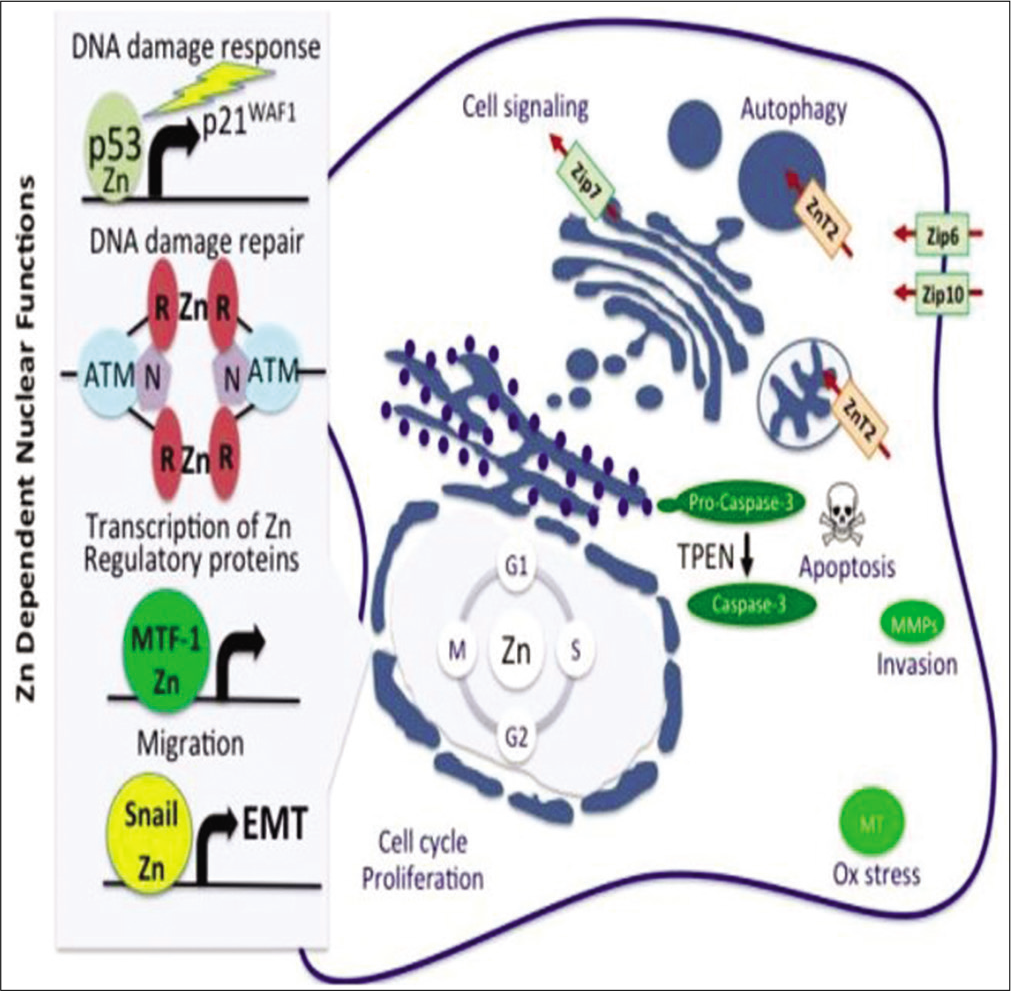
- Molecular mechanism of zinc antidotal effects, including oxidative stress abolition. (Source: World Health Report, 2002).[52]
In addition, Zn is a potent physiological regulator of the signal transduction of a key hormone in glucose homeostasis, insulin. It does this by its inhibitory action on protein tyrosine phosphatase 1β, the major phosphatase that dephosphorylates the insulin receptor.[55] Optimum supply of Zn is important for adequate insulin synthesis to combat hyperglycemic states. Thus, Zn deficiency contributes to diabetic complications, which may include elevated susceptibility to infections from compromised immunity (immunosuppression), accelerated generation of inflammatory cytokines, and of course oxidative stress. Experimental studies have provided evidence that supplementation of 5 mg/kg ZnSO4 attenuates DM-associated renal oxidative pathology, including inflammation as well as prevents the kidney from DM nephropathy; characterized by proteinuria and other pathological derangements.[58] This again appears to be corroborative of the need to include this seminal micronutrient and antioxidant in the traditional biochemical panels including that of DM.
RENAL DISEASE AND TOXIC STATES
In renal diseases, more zinc is lost through the kidneys than normal. In renal failure, for instance, excess zinc is lost through the dialysate when patients are maintained on regular dialysis treatment. Indeed, it has been suggested that children with an anephric condition on dialysis must be monitored with sexual maturity; zinc supplementation can reverse the symptoms.[59] The antioxidant enzymes or small molecular compounds are distributed in the body in an organ or system-dependent manner. Several organs such as the liver and the kidney are rich in high antioxidant concentration unlike other organs such as the heart, skeletal muscle, and the central nervous system.[60] This may also in part be a result of the detoxification role of these organs in toxic states.
Several studies have suggested that an important mechanism in kidney injury and disease, particularly in toxic kidney injury, involves oxidative stress.[61] Thiols (-SH groups), especially GSH, are particularly important in this. Depletion of thiols of intracellular GSH predisposes to kidney disease, particularly proximal tubular oxidative stress. This is particularly significant in that the PCT is important for inactive Vitamin D production with its effect on calcium homeostasis. Other antioxidants such as Vitamins C and E have been reported to be in the kidney of experimental models, particularly those exposed to nephrotoxicants.[62] The activity of several antioxidant systems has been reported to be markedly reduced post-exposure to nephrotoxic agents. Studies indicate that exposure to mercuric chloride caused a marked reduction in the levels of some key antioxidants such as superoxide dismutase, catalase, GSH peroxidase, and GSH disulfide reductase in the cortex of the kidney.[63] Reduction in these antioxidant defense systems will enhance the susceptibility of the kidney to many diseases, especially those associated with oxidative damage. These appear to be reasons supporting incorporating the antioxidant hypothesis in the assessment of renal disease or traditional profile of kidney disease, contributing to better risk assessment and therapy.
Some investigators have indicated that an intimate relationship exists between thiobarbituric acid reactants (TBARS), markers of oxidative stress, cellular redox status, maintenance of normal renal function, the renal cellular concentration of GSH, and the generation of biological energy (ATP) in the mitochondria. These relationships may be the basis of the investigation by Lund et al.[64] to study the role of a toxicant such as Hg-induced oxidative stress in the mitochondria of the kidney, especially as regards H2O2. These have been examined in detail by Lund et al.[64] Further studies have suggested that Fe-dependent lipid peroxidation was increased nearly 4-fold at the NADH dehydrogenase area and by 25% at the ubiquinone-cytochrome-b location. Intracellular mitochondrial, GSH is known to be decreased in duration and time-dependent fashion by toxicants such as HgCl. A 12 nmol/mg protein concentration of this toxicant completely used up mitochondrial GSH within half an hour, suggesting that mitochondrial oxidative stress is rapidly induced by exposure to common environmental toxicants such as compounds of Hg. In addendum, Lund et al.[65] further demonstrated that the production of H2O2, lipid peroxidation, and the consequent depletion of GSH were all markedly increased in the mitochondria removed from kidney cortical homogenates of animal models exposed to in vivo HgCl, with appropriate respiratory constituents. These data collectively suggest that toxicant-induced oxidative stress within the mitochondria may be an important mechanism of renal tubular injury common in several kidney diseases. This observation probably indicates the need to examine the antioxidant system employing markers of oxidative stress along with traditional panels for the assessment of renal function. The importance of antioxidant profiling is to identify potential markers of risk assessment, shortening the pathway to diagnosis, and disease management or therapy. The potential toxicity of ROS is counteracted by a large number of cell-protective enzymes, which forestall or limit the damage that could be caused by free radicals thus, offering a protective mechanism. The scavenging system, which protects against the toxic effects of free radicals when deficient, may lead to free radical-induced toxicity which can be detected in a modified traditional profile. The realization of the role of oxidative stress in the pathogenesis of a wide range of clinical conditions appears to make it instructive that this will be followed by therapy to minimize the toxic effect of the etiological ROS, preceded by a testing process. Indeed, Cooke et al.[6] had observed over one and a half decades ago that once the analytical system for oxidative stress assessment is well established, it should be possible to use oxidative DNA damage (a specific case of oxidative stress) “measurements as a clinical parameter, to more closely monitor treatments, identify at-risk populations, and ultimately device prevention strategies.” Although the therapeutic aspect may not have been implemented exactly as envisaged, the current use of supplements of all varieties appears to have been a fulfillment of that expectation.
THRESHOLD OF CLINICAL CONCERN
Laboratory science or medicine is involved in risk assessment, though poorly appreciated in diagnosis and treatment. Risk assessment in science is an extremely important field with great and far-reaching significance.[66] Incorporating markers of oxidative stress have the benefit of improving the current traditional process of disease recognition, detection, and therapy. The current traditional panels in clinical chemistry may be compared to toxicological risk assessment which has historically relied on the “monotonic dose-response relationship” of Paracelsus.[67] Colacci and Kleinstreuer[67] have recently called for a rethink of the process of risk assessment by incorporating low-dose exposure, particularly in chemical mixtures just as we are advocating here to add value to laboratory medicine. Like these scientists have argued for toxicological risk assessment, the time has also come for scientists and practitioners in the field of laboratory science and medicine to re-examine the traditional panels in clinical chemistry. This may help in more pragmatically discerning a healthy state from adaptive response to disease and a potential true pathological state as well as guide therapy.[68] As biomarkers of oxidative stress are mechanistic endpoints, they have the potential of integrated biological assessment and reducing the uncertainty inherent in current panels enabling us to respond to clinical needs and add value to laboratory medicine, a current goal of the International Federation of Clinical Chemistry, probably leading to new thresholds of clinical concern. Anetor et al.[69] have provided evidence of the depressing antioxidant status and pathologic correlates in pregnant women on Fe supplement, corroborating the promise and benefit of including markers of oxidative stress in traditional profiles in clinical chemistry. Jayasena et al.[70] have provided evidence of reduced testicular steroidogenesis and increased semen oxidative stress in male partners as novel markers of recurrent miscarriage. These investigators raised the possibility of seminal ROS measurement as diagnostic and therapeutic in couples with recurrent pregnancy loss. Although the observation of Jones et al[71] only highlighted the negative aspects of complementary and alternative medicine and by extension, the antioxidant hypothesis, they at least agreed that it is based on sound scientific principles. Most of the allegations and fears they raised in their review may have been largely addressed by an earlier commentary of Stohs and Preuss,[72] wherein they discussed what healthcare professionals should understand about the regulation and safety of supplements.
SUPPLEMENTS AND BIOTHERAPY
It may not be out of place in concluding this brief discussion to make some brief remarks on biotherapy, defined as a type of treatment that involves the use of substances derived from living organisms to mitigate or treat disease. These substances may be found in the body such as endogenous antioxidants such as GSH or synthesized. Biotherapy may in part involve stimulation of or suppression of the immune system as in cancer treatment to aid the body in combating the disease. Holford et al.[14] have examined the use of ascorbic acid as adjuvant therapy for respiratory infection, sepsis, and COVID-19. They remarked that arising from the favorable and cheap nature of ascorbic acid and the frequency of deficiency in many diseases including respiratory infections, it appears rational to test for patients’ Vitamin C levels and probably treat them based on the levels observed with intravenous infusion of Vitamin C for those in ICU and administered orally in hospitalized patients with COVID-19 for instance. Prasad from his lifelong focus on zinc[56] suggested that Zn therapy might be quite useful in chronic diseases. He maintains that zinc supplementation improves CMI, decreases oxidative stress, and lowers the generation of inflammatory cytokines in humans. Prasad[56] also opines that those sensitive immunological biomarkers may be more sensitive than plasma Zn and peripheral blood cells for diagnosis of marginal but significant Zn deficiency in humans. Rosenkranz et al.[73] have observed that zinc enhances the number of regulatory T cells in allergen-stimulated cells from atopic subjects.
Flora et al.[74] have examined alterations of physiological processes in lead toxicity, through oxidative mechanisms and the modulating of thiol groups as protective agents against ROS, as therapeutic intervention employing antioxidants. Ugwuja et al.[75] have also demonstrated that Zn mitigates the deleterious effects of Pb including associated hematological and biochemical derangements. The report of Robitaille and Hoffer[76] has highlighted the prevalence of hypovitaminosis C or marginal Vitamin C deficiency in contemporary society and the wide-ranging clinical implications in the general population in many common clinical and social states and justification for the determination of this key antioxidant vitamin in hospitalized and ambulant patients, a situation that remains almost completely unrecognized. Although some continue to argue about the clinical relevance of oxidative stress in clinical practice,[71] the report of Robitaille and Hoffer[76] and that of Adedapo et al.[77] found that women with decreasing consumption of antioxidants were more likely to develop preeclampsia. This supports oxidative stress as a factor in the pathogenesis of this clinical condition lending credence to the importance of oxidative stress in clinical practice and the need to include markers of oxidative in clinical chemistry repertoire.
CONCLUSION
Over two decades ago, it was thought that “the better understanding of the role of ROS in the pathogenesis of diseases, primarily as an indicator of disease and second as a consequence of disease, was likely to lead to the development of a variety of drugs to counter these radical activities in vivo.” It was also thought that this expectation will give rise to the prospect of disease prevention and effective treatment of disease. Rationally, this would have implied that at least markers of oxidative stress would have been incorporated into the core testing process in chemical pathology, which has fallen short of expectation. It is, therefore, proposed that redefining traditional profiles in chemical pathology by incorporating aspects of the antioxidant system will considerably improve the risk assessment process in disease detection and management. Evolving evidence suggests that the time has come for the scientific community to put the fruits of decades of research in the field of antioxidants into the routine chemical pathology practice, as already done in therapeutics. Many people today inadvertently take supplements for prophylaxis and adjunct therapy based at least in part on the antioxidant hypothesis. It is suggested that this be extended to clinical chemistry; an important component of the disease diagnosis and therapy ecosystem system.
Declaration of patient consent
Patient consent is not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Uses of Biochemical Data in Clinical Medicine Edinburgh: Churchill Livingstone; 2014. p. :1-5.
- [CrossRef] [Google Scholar]
- Clinical Biochemistry: An Illustrated Color Text (5th ed). Edinburgh: Churchill Livingstone; 2013. p. :2-3.
- [Google Scholar]
- The definition and measurement of antioxidants in biological systems. Free Radic Biol Med. 1995;18:125-6.
- [CrossRef] [Google Scholar]
- Free radicals, oxidative stress, and antioxidants in human health and disease. J Am Oil Chem Soc. 1998;75:199-212.
- [CrossRef] [PubMed] [Google Scholar]
- Antioxidant in health and disease. J Clin Patholo. 2001;54:176-86.
- [CrossRef] [PubMed] [Google Scholar]
- Does measurement of oxidative damage to DNA have clinical significance? Clin Chim Acta. 2006;365:30-49.
- [CrossRef] [PubMed] [Google Scholar]
- Cellular defense system gene expression profiling of human whole blood: Opportunities to predict health benefits in response to diet. Adv Nutr. 2012;3:499-505.
- [CrossRef] [PubMed] [Google Scholar]
- Toxic effect of Metals In: Fundamentals of Toxicology; Essential Concepts and Applications (1st Edition). 2016. p. :203-213.
- [CrossRef] [Google Scholar]
- Clinical relevance of biomarkers of oxidative stress. Antioxid Redox Signal. 2015;23:1144-70.
- [CrossRef] [PubMed] [Google Scholar]
- Nutritional antioxidants in cancer, other degenerative and toxic states In: Farombi EO, ed. Nutritional Antioxidants in Cancer and Degenerative Diseases. Fort Kerala, India: Research Signpost; 2009. p. :37-66.
- [Google Scholar]
- Clinical chemistry laboratory automation in the 21st century-Amat Victoria Curam (Victory loves careful preparation) Clin Biochem Rev. 2014;35:143-53.
- [Google Scholar]
- Why the albumin to creatinine ratio should replace protein to creatinine ratio: It is not just about nephrologists. Ann Clin Biochem. 2013;50:301-5.
- [CrossRef] [PubMed] [Google Scholar]
- The role of oxidative stress and antioxidant in liver disease. Int J Mol Sci. 2015;16:26087-124.
- [CrossRef] [PubMed] [Google Scholar]
- Vitamin C-an adjunctive therapy for respiratory infections, sepsis, and COVID-19. Nutrient. 2020;12:37060.
- [CrossRef] [PubMed] [Google Scholar]
- Measuring reactive species and oxidative damage in vivo and in cell culture: How should you do it and what do the results mean? Br J Pharmacol. 2004;142:231-55.
- [CrossRef] [PubMed] [Google Scholar]
- Oxidative stress and non-alcoholic fatty liver disease: Effects of omega-3 fatty acid supplementation. Nutrient. 2019;11:872.
- [CrossRef] [PubMed] [Google Scholar]
- Free radicals, antioxidants and human disease: Where are we now? J Lab Clin Med. 1992;119:598-620.
- [Google Scholar]
- Redox imbalance in the critically ill. Br Med Bull. 1999;55:49-75.
- [CrossRef] [PubMed] [Google Scholar]
- Plasma levels of antioxidant vitamins in relation to ischemic heart disease and cancer. Am J Clin Nutr. 1987;45(Suppl 5):1368-77.
- [CrossRef] [PubMed] [Google Scholar]
- Vitamins and Minerals in Health and Nutrition Chichester, England: Ellis Horwood Ltd.; 1990.
- [Google Scholar]
- Glutathione: New roles in redox signaling for an old antioxidant. Front Pharmacol. 2014;5:196.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative analysis of the antioxidative and hepatoprotective activities of dimethyl diphenyl bicarboxylate in four animal models of hepatic injury. Antioxidants. 2021;10:1508.
- [CrossRef] [PubMed] [Google Scholar]
- Iron, oxidative stress and human health. Mol Aspects Med. 2005;26:299-312.
- [CrossRef] [PubMed] [Google Scholar]
- Increased oxidation and decreased conjugation of drugs in the liver caused by starvation; Altered metabolism of certain aromatic compounds and acetone. Chem Biol Interact. 1995;96:87-101.
- [CrossRef] [Google Scholar]
- Assessment of hepatic function and investigation of jaundice In: Marshall WJ, Lapsley M, Day PA, Ayling RM, eds. Clinical Biochemistry: Metabolic and Clinical and Metabolic Aspects. Edinburgh: Churchill Livingstone; 2014. p. :231-49.
- [CrossRef] [Google Scholar]
- Glutathione, ascorbate, and cellular protection. Cancer Res. 1994;54(Supp1):1969S-75.
- [Google Scholar]
- Glutathione synthetase deficiency and other disorders of the gamma-glutamyl cycle In: Scriver CR, Beaudet alSly WS, Valle D, eds. The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw-Hill; 1995. p. :1461-95.
- [Google Scholar]
- Mitochondrial changes associated with glutathione deficiency. Biochim Biophys Acta. 1995;1271:35-42.
- [CrossRef] [Google Scholar]
- Glutathione, ascorbic acid and antioxidant enzymes in the tumor tissue and blood of patients with oral squamous cell carcinoma. Eur Rev Med Pharmacol Sci. 2005;9:361-7.
- [Google Scholar]
- Glutathione and glutathione delivery compounds. Adv Pharmacol. 1997;38:65-78.
- [CrossRef] [Google Scholar]
- Glutathione improves the antioxidant activity of Vitamin C in human lens and retinal epithelial cells: Implications for vitreous substitutes. Curr Eye Res. 2021;46:470-81.
- [CrossRef] [PubMed] [Google Scholar]
- Oxidative stress and diabetic cardiovascular complications. Free Radic Biol Med. 2006;40:183-92.
- [CrossRef] [PubMed] [Google Scholar]
- The role of oxidative stress in the pathogenesis of diabetic vascular complications. Diabetes Metab J. 2012;36:255-61.
- [CrossRef] [PubMed] [Google Scholar]
- A negative association between erythrocyte reduced glutathione concentration and diabetic complications. Clin Sci (Lond). 1996;91:575-82.
- [CrossRef] [PubMed] [Google Scholar]
- The pathobiology of diabetic complications: A unifying mechanism. Diabetes. 2005;54:1615-25.
- [CrossRef] [PubMed] [Google Scholar]
- Superior renoprotective effects of combination therapy with ACE and AGE inhibition in the diabetic spontaneously hypertensive rat. Diabetologia. 2004;47:89-97.
- [CrossRef] [PubMed] [Google Scholar]
- Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev. 2004;25:612-28.
- [CrossRef] [PubMed] [Google Scholar]
- Nitrosative injury and antioxidant therapy in the management of diabetic neuropathy. J Investig Med. 2004;52:33-44.
- [CrossRef] [Google Scholar]
- Fragmentation of ceruloplasmin following non-enzymatic glycation reaction. J Biochem (Tokyo). 1995;118:1054-60.
- [CrossRef] [PubMed] [Google Scholar]
- Biological chemistry of thiols in the vasculature and in vascular-related disease. Nutr Rev. 1996;54:1-30.
- [CrossRef] [PubMed] [Google Scholar]
- Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995;11:376-81.
- [CrossRef] [PubMed] [Google Scholar]
- Nitric oxide and oxidative stress in vascular disease. Pflugers Arch. 2010;459:923-39.
- [CrossRef] [PubMed] [Google Scholar]
- Roles of glutathione peroxidase in the protection against endothelial cell injury induced by 15-hydroperoxy-eicosatetraenoic acid. Arch Biochem Biophys. 1992;294:407-11.
- [CrossRef] [Google Scholar]
- Oxidative stress in acute and chronic pancreatitis. Am J Clin Nutr. 1995;62(Suppl 6):1306S-14.
- [CrossRef] [PubMed] [Google Scholar]
- Acetylcysteine to treat complications of pancreatitis. Lancet. 1986;1:914-5.
- [CrossRef] [Google Scholar]
- Why has the mortality rate for severe malnutrition remained so high? Bull World Health Organ. 1996;74:223-9.
- [Google Scholar]
- Dietary reference values of individual micronutrients and nutriomes for genome damage prevention: Current status and a road map to the future. Am J Clin Nutr. 2010;91:1438S-54.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of inorganic nutrients on maintenance of genome stability, Environ Mol Mutagen. . 2009;50:349-60.
- [CrossRef] [PubMed] [Google Scholar]
- The World Health Organization Report Geneva, Switzerland: World Health Organization; 2002. p. :47-97.
- [Google Scholar]
- Scope and methods of prevention In: Last JM, Chin J, Fielding JE, Frank AL, Lashoff JC, Wallace RB, eds. MaxcyRosenau Public Health and Preventive Medicine. Norwalk, CT Connecticut: Appleton-Century-Crofts; 1986. p. :3-7.
- [Google Scholar]
- Mixed Chemical induced oxidative stress in occupational exposure. Afr J Biotechnol. 2009;8:821-6.
- [Google Scholar]
- Discovery of human zinc deficiency: Its impact on human health and disease. Adv Nutr. 2013;4:176-90.
- [CrossRef] [PubMed] [Google Scholar]
- Lessons learned from an experimental human model of zinc deficiency. J Immunol Res. 2020;2020:9207279.
- [CrossRef] [PubMed] [Google Scholar]
- Zinc and diabetes In: Rink L, ed. Zinc in Human Health. Netherland: IOS Press; 2011. p. :498-513.
- [Google Scholar]
- Zinc decrease-reactive protein, lipid peroxidation, and inflammatory cytokines in elderly subjects: A potential implication of zinc as an atheroprotective agent. Am J Clin Nutr. 2010;91:1634-41.
- [CrossRef] [PubMed] [Google Scholar]
- Role of membrane lipids in metabolic regulation. Nutr Rev. 1988;46:145-9.
- [CrossRef] [PubMed] [Google Scholar]
- Diclofenac-induced liver injury: A paradigm of idiosyncratic drug toxicity. Toxicol Appl Pharmacol. 2003;192:307-22.
- [CrossRef] [Google Scholar]
- Oxidative stress is a major culprit in kidney disease in diabetes. Diabetes. 2003;57:1446-54.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of zinc pretreatment on mercuric chloride-induced lipid peroxidation in the rat kidney. Toxicol Appl Pharmacol. 1984;73:395-401.
- [CrossRef] [Google Scholar]
- Glutathione depletion and in vitro lipid peroxidation in mercury or meleate-induced acute renal failure. Biochem Pharmacol. 1983;32:69-72.
- [CrossRef] [Google Scholar]
- Mercury-induced H2O2 production and lipid peroxidation in-vitro in rat kidney mitochondria. Biochem Pharmacol. 1991;42(Suppl):S181-7.
- [CrossRef] [Google Scholar]
- Studies on Hg (II)-induced H2O2 function and oxidative stress in vivo and in-vitro in rat kidney mitochondria. Biochem Pharmacol. 1993;45:2017-24.
- [CrossRef] [Google Scholar]
- Toxicity and Risk-Context, Principles and Practice. London: Taylor and Francis; 2001
- [CrossRef] [Google Scholar]
- Measurement and clinical significance of oxidative stress in humans. Oxid Med Cell Longev. 2017;2017:6501046.
- [CrossRef] [PubMed] [Google Scholar]
- Depressed antioxidant status in pregnant women on iron supplements: Pathologic and clinical correlates. Biol Trace Elem Res. 2010;136:157-70.
- [CrossRef] [PubMed] [Google Scholar]
- Reduced testicular steroidogenesis and increased semen oxidative stress in Male partners as novel markers of recurrent miscarriage Clin Chem. . 2019;65:161-9.
- [CrossRef] [PubMed] [Google Scholar]
- Laboratory tests commonly used in complementary and alternative medicine: A review of the evidence. Ann Clin Biochem. 2019;56:310-25.
- [CrossRef] [PubMed] [Google Scholar]
- What health care professionals should know about the regulation and safety of dietary supplements. J Am Coll Nutr. 2017;36:306-9.
- [CrossRef] [PubMed] [Google Scholar]
- Zinc enhances the number of regulatory T cells in allergen-stimulated cells from atopic subjects. Eur J Nutr. 2017;56:557-67.
- [CrossRef] [PubMed] [Google Scholar]
- Toxicity of lead: A review with recent updates. Interdiscipl Toxicol. 2012;5:47-58.
- [CrossRef] [PubMed] [Google Scholar]
- Zinc ameliorates lead toxicity by reducing Pb burden and restoring Pb-induced hematological and biochemical derangement. Toxicol Res Appl. 2020;4:1.
- [CrossRef] [Google Scholar]
- A simple method for plasma total Vitamin C analysis, suitable for clinical laboratory use. Nutr J. 2016;15:40.
- [CrossRef] [PubMed] [Google Scholar]
- Increased oxidative stress in Nigerian women with preeclampsia: Scientific letter. S Afr J Obstet Gynaecol. 2011;17:50-1.
- [Google Scholar]







