Translate this page into:
Inflammatory responses and obesity: Nutrition as an epigenetic modulator

*Corresponding author: Debasis Bagchi, College of Pharmacy and Health Sciences, Texas Southern University, Houston, Texas, United States. debasisbagchi@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Bagchi D, Downs BW, Banik SP, Chakraborty TR, Chakraborty S, Kushner S. Inflammatory responses and obesity: Nutrition as an epigenetic modulator. Am J Biopharm Pharm Sci 2022;2:9.
Abstract
The onset of inflammation takes place in a human body due to an injury or infection during which the tissue becomes inflamed/reddened, swollen, hot, and painful. Basically, it is a collection of host defenses that occurs during an injury and infection in which the white blood cells protect the body from infection from bacteria, fungi, parasites, or viruses. Innate immunity provides the first challenging defense against the diverse foreign harmful invaders, while adaptive immunity, also known as acquired immunity, utilizes specialized immune cells and antibodies, which provide a counterattack and destroy these diverse foreign invaders. Moreover, they can prevent infections/diseases in the future by recognizing those invaders and providing a new immune response. However, when an immune system responds too aggressively to an infection, a condition termed a cytokine storm takes place, which may lead to multi-organ failure and even death. Inflammatory response in advancing age and obesity is intricately associated. Obesity has been identified as a low-grade systemic inflammatory response. Particularly, elevated levels of serum C-reactive protein, interleukin-6, tumor necrosis factor-α, and leptin, well characterized biomarkers of inflammation, are observed predominantly in obese individuals.
Keywords
Inflammation
Immunity
Infection
Cytokine storm
Advancing age
Obesity
Graphical Abstract
INFLAMMATION: AN INTRODUCTION
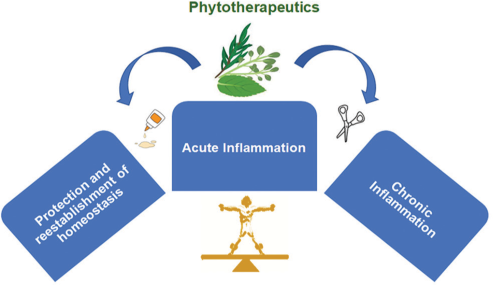
It is well established that that there are two kinds of inflammatory responses, namely, (1) acute inflammation and (2) chronic inflammation. In response to an injury in a human body, reddening of the skin, warm sensation, tissue swelling, and pain occurs surrounding the skin tissues/ joints, while the body’s immune support system releases white blood cells, termed leukocytes, to encircle and protect the injured area in speeding up the healing process.[1-3] Thus, acute inflammation initiates an organizes a cascade of events strategized to maintain tissue and organ homeostasis. This inherent process releases mediators and expression of receptors, which are quite mandatory and vital to restore tissues to their primal condition. Thus, inflammation is quite beneficial to repair/regenerate/restructure the injured tissue[1,4] [Figure 1].

- An overview of acute inflammation as a necessary evil in the body: Elicitation of acute inflammatory response thwarts the attack of pathogens and re-establishes homeostasis, whereas dysregulation in the same with prolonged persistence of pro-inflammatory mediators leads to chronic inflammatory response with severe and long-term ailments.
INFLAMMATION AND IMMUNE COMPETENCE
When the inflammatory response lingers and continues for a longer time, while the immune support system continues to provide white blood cells and multiple chemical messengers that lengthen the process, it is termed chronic inflammation.[1,5,6] Under these lengthy stressful conditions, white blood cells get distracted/misguided and finally invade the adjoining healthy cells, tissues, and organs. Chronic and low-grade inflammation becomes a silent killer that contributes to an array of diverse degenerative disorders including cardiovascular disease, arthritis, type 2 diabetes mellitus (T2DM), metabolic distress/syndrome, inflammatory bowel diseases (Crohn’s disease) and ulcerative colitis, cancer, and diverse infectious conditions/diseases.[4,5,7]
INNATE IMMUNITY AND ADAPTIVE IMMUNITY
It is well established that immune cells in a human body can invade pathogens including bacteria, fungi, and viruses, as well as other foreign elements by two intricate mechanisms of (a) immediate recognition or (b) priming for recognition, a novel process of priming the immune cells for recognition of pathogens. An immune system is comprised of (i) innate immunity, namely, inborn immunity which provides the first line of defense against diverse injury and pathogenic infections, and (ii) adaptive immunity, which is acquired, or primed immune competence and is generally triggered afterwards.[5,8]
The immediate recognition mechanism primes the cells of the innate immunity network to recognize/identify/perceive microbial danger associated with the pathogen(s), which, in turn, immediately bind the pathogens to the receptors on the immune cells. This binding creates a complex signaling cascade and organizes a massive supply of immune cells in the vicinity, which, in turn, envelope the pathogens or institute the production of mediators that initiate the destruction of pathogens. Priming for recognition is a secondary exercise that utilizes cells of the adaptive immune system.[7-9] These cells are required to be “primed to detect” and differentiate the foreign invader(s) from self. As a matter of fact, priming takes place when portions/fragments from pathogenic elements are processed by selected cells to prompt the production of suitable receptors for these pathogens on adaptive immune cells. Subsequently, when these adaptive immune cells differentiate and multiply by the very same pathogen, this priming for the recognition process validates the adaptive immune cells to identify the pathogen and institute an invasion on it or invigorate the enhanced creation of antibodies against the pathogen.[8,9]
This pathogenesis establishes the basis of the defending granted by immunization. Importantly, both the innate and adaptive immune systems largely depend on the identification, differentiation, and characterization of the “non-self ” from “self ” antigens and on their capacity to actuate mediators, which, in turn, can induct a larger reservoir of immune cells.[1,2] It is important to emphasize that these mediators are basically inflammatory mediators which, in turn, try to attract immune cells to the vicinity of injury/ infection and to damaged/injured/infected cells/tissues. This typical physiological process is termed inflammation, which occurs in response to injury and/or infections that steer the pathogen eradication process along with tissue repair/ regeneration/restructuring process to restore homeostasis at the injured/infected sites.[5]
Ironically, a magnified or overemphasized response by the innate immune system may steer to both chronic inflammation and autoimmune response. As indicated earlier, chronic inflammation leads to a remarkable number of degenerative diseases, while autoimmune distress emanates from non-recognition of self-antigenic peptides, causes asthma, rheumatoid arthritis (RA), psoriasis, psoriatic arthritis, lupus, T1DM, and thyroid diseases[7,8] [Figure 2].

- Molecular basis of dysregulation of immune response in the autoimmune disorder Rheumatoid Arthritis (RA): Autoantibodies against synovial tissue fluid proteins rheumatoid factors and citrunillated peptides cause priming of immune response as these arthritis specific antigens are internalized and processed by the Antigen Presenting Cells (B cells, macrophages and dendritic cells) and subsequently presented to the CD4+ TH cells in the vicinity. This causes differentiation of TH cells mainly into TH1 cells which in turn secrete a battery of pro-inflammatory cytokines including TNFα and IFNγ. In RA, the other subpopulation of TH2 cells is not produced significantly due to an impaired differentiation pathway. This causes a substantial drop in the concentration of the anti-inflammatory cytokines which would otherwise bring down the immune response. This heightened immune response is augmented by a steep rise in the ratio of TH17: Treg cells which further boosts the concentration of pro-inflammatory cytokines. An almost non-functional Treg response (which would otherwise dampen the immune response by preventing differentiation of TH cells) leads to elicitation of cytokine storm with recruitment of NK (Natural Killer) cells, upregulation of MMPs (matrix metalloproteases) and a host of other destruction machineries. This causes the pathophysiology associated with RA.
Taken together, inflammation-immune response is becoming increasingly important to protect from infection and injury. Thus, inhibition/intervention processes are becoming increasingly important in the immune-inflammation processes at the cellular and molecular level.[9,10]
CYTOKINE STORM
When the immune system responds too aggressively to an infection, a condition termed cytokine storm or cytokine-associated toxicity or cytokine release syndrome (CRS) takes place[11,12] [Figure 2]. Cytokines are integral constituents of a healthy immune system, which regulates the growth and activity of blood and immune cells. Cytokines regulate the functions of the immune system; however, release of too many cytokines jeopardizes compromised immune, resulting in a cytokine storm. However, this situation may also take place after certain types of immunotherapies or chemotherapies, known as chimeric antigen receptor T-cell therapy, a procedure of utilizing T cells to destroy cancer cells. During CRS, the human body releases cytokines and exerts multiple different symptoms including high fever, multi-organ toxicity, and other noxious manifestations, which, in turn, can affect multiple organs. A cytokine storm exerts a variety of symptoms including fever, cough, lethargy, difficulty in swelling, dizziness, nausea, vomiting, low blood pressure, pulmonary damage, joint pain, delirium, edema, skin rash, and muscle pain. Thus, CRS may damage multiple organs simultaneously and may cause multi-organ failure leading to death.[11,12]
The cytokine network in RA is a complex field, with a lot of cytokines showing pleiotropic actions and many different targets. Interestingly, cytokine blockade has been demonstrated to be a new strategy for treating RA patients, while tumor necrosis factor-α (TNF-α) and interleukin-1 beta (IL-1β) have been demonstrated to play a vital role.[11,12] Dr. Fox demonstrated that the most pronounced abundance of TNF and IL-1β in the list of cytokines was detected in the synovial tissue or fluid in RA patients.[11] An effective way to reduce TNFα in RA patients is to incorporate soluble TNF receptor(s) or anti-TNF monoclonal antibodies that seems to be an effective way of regulating RA disease progression.[11,12] Dr. Fox further emphasized that importance of long-term safety of TNF blockade should be investigated and its importance on disease pathogenesis.[11]
INFLAMMATION, ADVANCING AGE, AND OBESITY
We shall now address the relationship between inflammation, advancing age, and obesity. Researchers have demonstrated obesity as a low-grade systemic inflammatory disorder. Especially, obese individuals exhibit elevated levels of serum C-reactive protein (CRP), IL-6, TNF-α, and leptin, well characterized biomarkers of inflammation and diverse degenerative disorders.[13,14] There are several factors contributing to obesity: (a) Poor dietary habits, namely, overindulgence of food, high-fat diet, sugar, and salty foods; (b) inadequate physical activity/sedentary lifestyle; (c) exposure to environmental toxins, agribusiness practices, and livestock treatment; (d) processed foods, food additives, flavor enhancers, preservatives, and colorants; (e) digestive malfunction and dysbiosis, which, in turn, boosts survival panic, impairs gut-brain interconnectivity, impairs cognition, elevates stress intolerance, depression, binge eating, enhanced energy conservation, and fat storage; (f) increases anaerobic metabolism (cannot effectively utilize O2 and H2O); and (g) increases inflammatory responses leading to stubborn fat accumulation. Fat tissue itself acts as an organ and sends out chemical messengers that inhibit healthy fat metabolism, increases fat storage, and retards protein conversion into muscle. The cascade of events takes place including (a) enhanced oxidative stress and increased oxidative tissue damage, (b) increased immune system distress and exhaustion, which in turn can also trigger ‘survival panic’; (c) premature aging; and (d) impaired aerobic metabolism, causing increased anaerobic glycolysis and metabolism, and drastically reducing AMPK levels, which is termed the energy coin.[5-8]
Accumulation of atypical and uncontrolled growth of excessive fat is termed obesity, which interferes with the maintenance of healthy metabolism. The longer an individual is obese, the longer the body will exhibit a state of inflammation. Especially, overweight and obese individuals have visceral fat tissues/cells circumambient of multiple vital organs. Immune cells consider these cells a threat to the living organism and in turn invade them along with white blood cells.[8,9]
Adipose and hepatic tissues participate in maintaining lipid and glucose homeostasis and play a pivotal role in nutrient uptake, processing, transportation, and storage, while selected nutrients exert potential influences on the liver-adipose tissue axis. Importantly, macronutrient metabolism is a unique organized process, where hepatic and adipose tissues play a pivotal role in nutrient uptake, transportation, and processing.[1,9,10] However, excessive macronutrient storage in the adipose tissues triggers them to generate inflammatory mediators including TNFα and IL-6, suppressing the production of adiponectin, leading to a pro-inflammatory state. This in turn induces oxidative stress and enhances production of reactive oxygen species. Furthermore, IL-6 triggers the hepatic tissues to release CRP. Ultimately, in overweight and obese individuals, adipose tissue becomes dysfunctional, and thus, an excessive nutritional status induces a potential risk for obesity and fatty liver diseases.[7,8]
It is important to elaborate how adversarial gene expression of neurotransmitters and hormones are that are involved in fat metabolism. These include (i) leptin resistance, which can result in and/or exacerbate infertility, profound obesity, T1DM, T2DM, and other insulin resistant disorders in humans, that is, Rabson-Mendenhall Syndrome; (ii) ghrelin, the hunger hormone, which is a survival panic-induced hormone that increases appetite level, promotes cell longevity and tissue protection; and (iii) reproductive hormones. Adipose tissue-derived estrogens exert anti-inflammatory activities in the periphery and central nervous system to protect from inflammation. The hypothalamus regulates energy homeostasis in the ventrolateral region of the ventromedial nucleus and the arcuate fibers respond to hormones and other signals to regulate energy homeostasis, namely, energy intake and expenditure. Thyroid hormones T3 and T4 play a key role in body weight regulation and energy homeostasis. The neurotransmitters serotonin and dopamine are involved in the regulation of food cravings and energy homeostasis.[9-11]
FUTURE STRATEGIES FOR REGULATING OBESITY
In recaping the paradigm of this editorial, it is important to understand that weight loss is an unreliable metric. Placing a primary focus on the heaviness of the body or overweight does not provide an accurate perspective for determining healthy changes in metabolism, body composition, or size of the individual.[6] Fat is the lightest macromolecule in the human body and the last to be eliminated in the body recomposition process. Thus, short-term expectations in weight loss are erroneous. Furthermore, the body is genetically preprogrammed to survive and has three forms of survival insurance, which are (i) fat, (ii) sugar as glycogen, and (iii) water. When the body is put into a survival mode, that is, survival panic, it upregulates survival insurance storage of these components, retards energy expenditure, and increases energy conservation, and brown and white adipose tissue deposition.[6,9]
To combat the skyrocketing progression of obesity and metabolic syndrome around the world, it is important to explore different strategies of utilizing appropriate natural phytotherapeutics [Table 1] in conjunction with regular exercise and appropriate diet to combat obesity, metabolic syndrome, and attain a healthy and balanced lifestyle.[6]
| Ingredient | Chemical biomarkers | Molecular mechanisms |
|---|---|---|
| D-Ribose nicotinamide complex (RiaGev®)[15] | A proprietary blend of D-ribose and nicotinamide |
|
| Gynostemma pentaphyllum leaf extract[16] | Contains damulin A and B, two dammarane-type saponins |
|
| Myrciaria dubia (Camu Camu) extract (Nitro-C®)[17] | Contains considerable amounts of vitamin C, betulinic acid, β-carotene, riboflavin, thiamine, niacin, cyanidin 3-glucoside, delphinidin 3-glucoside, ellagic acid, kaempferol, myricetin, quercetin, quercitrin, rutin, and micronutrients |
|
| Ilex guayusa extract (Amatea®)[18] | Containing structurally diverse antioxidants, chlorogenic acid, ursolic acid, and natural caffeine containing supplement |
|
| Purple tea (Camellia sinensis extract) (Purple Force)[19,20] | High polyphenol and delphinidin containing antioxidant ingredients, especially 1,2-di-Galloyl-4,6-Hexahydroxydipheno yl–D-Glucose) that is not present in other GTEs |
|
| Alpha glyceryl phosphoryl choline (Alpha-GPC) (Alpha Size®)[21,22] |
A non-stimulating, safe and endurance enhancing ergogenic supplement |
Boosts endurance and performance, while preventing the reduction of choline levels |
An ideal strategy will be to incorporate these ingredients to ideally synergize the gut-brain axis and appropriately influence the human gut microbe associations for optimal body homeostasis and achieve the system biology benefits noted in the table.[23,24]
CONCLUSION
Two intricate molecular mechanisms adjudicate epigenetic modulation in a biological system, which are (a) DNA methylation and (b) histone modification. Especially, DNA methylation serves as a predominant role during advancing age.[25-27] Identification of proteins that mediate these effects has provided insight into this complex process and diseases that occur when it is perturbed. External influences on epigenetic processes are seen in the effects of diet on long-term diseases such as cancer.[25-28]
The popular vitamin folate plays a very predominant role in DNA methylation.[26,27] Normal growth in mammals is very much dependent on DNA methylation, while it is a biomarker in determining folate status, which is very much linked to chronic degenerative diseases including cancer, and responsible for disorders during development stage.[25-28] Furthermore, research has demonstrated the importance of nutrition during pregnancy and intake of betaine and methionine before and during pregnancy. There are many studies on DNA methyl transferases and leptin methylation, which exhibited that feeding folate reverses disrupted DNA methylation during cancer and other degenerative diseases.[26,27] Insufficient folate causes damage to the embryo and poses a longer-term risk for diabetes and metabolic syndrome.[26-28] On the contrary, elevated folate level and increased DNA methylation reduces the risk of cancer. Magueri and Barchitta have demonstrated the pronounced efficacy of folate, betaine, methionine, vitamins B2, B6 and B12, b-carotene, lycopene, retinol, lutein, and zeaxanthin in DNA methylation.[26]
Thus, epigenetic mechanisms seem to allow an organism to respond to the environment through changes in gene expression. The extent to which environmental effects can provoke epigenetic responses represents an exciting area of the future research.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. Debasis Bagchi is Associate Editor for the journal.
References
- Immunity and Inflammation in Health and Disease: Emerging Roles of Nutraceuticals and Functional Foods in Immune Support Boston, USA: Elsevier/Academic Pressl; 2018.
- [Google Scholar]
- Regulation of immunity and inflammation by hypoxia in immunological niches. Nat Rev Immunol. 2017;17:774-85.
- [CrossRef] [PubMed] [Google Scholar]
- Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front Cardiovasc Med. 2020;7:22.
- [CrossRef] [PubMed] [Google Scholar]
- Obesity and inflammation: The linking mechanism and the complications. Arch Med Sci. 2017;13:851-63.
- [CrossRef] [PubMed] [Google Scholar]
- Macronutrients and the adipose-liver axis in obesity and fatty liver. Cell Mol Gastroenterol Hepatol. 2019;7:749-61.
- [CrossRef] [PubMed] [Google Scholar]
- Effective body recomposition vs. Misconceptions of the traditional weight loss strategies: TRCAP21-a novel technological breakthrough in body recomposition. Funct Foods Health Dis. 2022;12:134-50.
- [CrossRef] [Google Scholar]
- Inflammation, Advancing Age and Nutrition: Research and Clinical Interventions Boston, USA: Elsevier/Academic Press; 2014.
- [Google Scholar]
- Chronic Inflammation: Molecular Pathophysiology, Nutritional and Therapeutic Interventions Boca Raton, FL: CRC Press/Taylor and Francis; 2013.
- [Google Scholar]
- Epigenetic remodeling in innate immunity and inflammation. Annu Rev Immunol. 2021;39:279-311.
- [CrossRef] [PubMed] [Google Scholar]
- Leptin in inflammation and autoimmunity. Cytokine. 2017;98:51-8.
- [CrossRef] [PubMed] [Google Scholar]
- Cytokine blockade as a new strategy to treat rheumatoid arthritis. Arch Intern Med. 2000;160:437-44.
- [CrossRef] [PubMed] [Google Scholar]
- Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785-8.
- [CrossRef] [PubMed] [Google Scholar]
- A combination of nicotinamide and D-Ribose (RiaGev) is safe and effective to increase NAD+ metabolome in healthy middle-aged adults: A randomized, triple-blind, placebo-controlled, cross-over pilot clinical trial. Nutrients. 2022;14:2219.
- [CrossRef] [PubMed] [Google Scholar]
- Antiobesity effect of Gynostemma pentaphyllum extract (Actiponin): A randomized, double-blind, placebo-controlled trial. Obesity (Silver Spring). 2014;22:63-71.
- [CrossRef] [PubMed] [Google Scholar]
- Antioxidant and associated capacities of Camu camu (Myrciaria dubia): A systematic review. J Altern Complement Med. 2015;21:8-14.
- [CrossRef] [PubMed] [Google Scholar]
- Guayusa (Ilex guayusa L.) new tea: Phenolic and carotenoid composition and antioxidant capacity. J Sci Food Agric. 2017;97:3929-36.
- [CrossRef] [PubMed] [Google Scholar]
- Multi-ingredient pre-workout supplements, safety implications, and performance outcomes: A brief review. J Int Soc Sports Nutr. 2018;15:41.
- [CrossRef] [PubMed] [Google Scholar]
- Purple tea and its extract suppress diet-induced fat accumulation in mice and human subjects by inhibiting fat absorption and enhancing hepatic carnitine palmitoyltransferase expression. Int J Biomed Sci. 2015;11:67-75.
- [Google Scholar]
- Glycerophosphocholine enhances growth hormone secretion and fat oxidation in young adults. Nutrition. 2012;28:1122-6.
- [CrossRef] [PubMed] [Google Scholar]
- Acute supplementation with alpha-glycerylphosphorylcholine augments growth hormone response to, and peak force production during resistance exercise. J Int Soc Sports Nutr. 2008;5(Suppl 1):15.
- [CrossRef] [Google Scholar]
- Signaling inflammation across the gut-brain axis. Science. 2021;374:1087-92.
- [CrossRef] [PubMed] [Google Scholar]
- Microbiota-brain-gut axis and neurodegenerative diseases. Curr Neurol Neurosci Rep. 2017;17:94.
- [CrossRef] [PubMed] [Google Scholar]
- Epigenetic modulation of DNA methylation by nutrition and its mechanisms in animals. Anim Nutr. 2015;1:144-51.
- [CrossRef] [PubMed] [Google Scholar]
- How dietary factors affect DNA methylation: Lesson from epidemiological studies. Medicina (Kaunas). 2020;56:374.
- [CrossRef] [PubMed] [Google Scholar]
- Folate and DNA methylation: A review of molecular mechanisms and the evidence for folate's role. Adv Nutr. 2012;3:21-38.
- [CrossRef] [PubMed] [Google Scholar]
- Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33(Suppl):245-54.
- [CrossRef] [PubMed] [Google Scholar]







