Translate this page into:
Complete Freund’s adjuvant-induced arthritis in rats: Anti-inflammatory and antioxidant properties of Allanblackia gabonensis (guttiferae) aqueous extract

*Corresponding author: Edwige Ymele Chiogo Vouffo, Department of Animal Biology, University of Yaounde I, Yaounde, Cameroon. edwige@umaryland.edu
-
Received: ,
Accepted: ,
How to cite this article: Chiogo Vouffo EY, Vouffo B, Donfack Vouffo E, Temdie RG, Akono EN, Azebaze A, et al. Complete Freund’s adjuvant-induced arthritis in rats: Anti-inflammatory and antioxidant properties of Allanblackia gabonensis (guttiferae) aqueous extract. Am J Biopharm Pharm Sci. 2023;3:7. doi: 10.25259/AJBPS_1_2023
Abstract
Objectives:
Allanblackia gabonensis (Guttiferae) stem bark extract is generally used in Cameroonian traditional medicine for its beneficial activities as antibiotics, anti-inflammatory, and antinociceptive. However, the claimed chronic anti-inflammatory and antioxidant effects have not yet been largely elucidated scientifically. The present study was designed to evaluate the anti-inflammatory and antioxidant effects of A. gabonensis stem bark aqueous extract on Freund’s complete adjuvant (CFA)-induced arthritis in rats.
Materials and Methods:
Arthritis was induced by intradermal injection of CFA (0.1 mL) into the right hind paw of each rat. Pain relieving effects were measured in the treated animals using an analgesiometer and antioxidant activity determined by measuring oxidative stress parameters. In addition, the hematological index, serum nitric oxide (NO), and transaminase activities were evaluated in the experimental animals.
Results:
A. gabonensis significantly protected animals against pain from day 15 to 18 and decreased (P < 0.01) the paw edema from day 12 to the end of the experimentation (day 22). The number of white blood cells increased while the NO levels in serum and organs decreased in CFA animals as compared to the control group. An increase in serum transaminases was observed in the CFA group. A. gabonensis at the dose of 200 mg/kg significantly increased (36.36%) glutathione levels in the spleen in comparison with the CFA group. There was also a significant increase (P < 0.01) of liver and cardiac catalase in animals receiving extract at 100 and 200 mg/kg.
Conclusion:
Our findings revealed the anti-arthritic and antioxidant potential of A. gabonensis and, thus, validate its traditional claim.
Keywords
Freund’s complete adjuvant
Allanblackia gabonensis
Anti-inflammatory
Antioxidant
Rat
INTRODUCTION
Freund’s complete adjuvant-induced arthritis in rats is one of the best models of chronic inflammation with an important pharmaceutical relevance for rheumatoid arthritis (RA).[1] RA is an example of those groups of diseases with chronic systemic disorders and it is considered an autoimmune disease with destructive inflammatory polyarticular joint potentially leading to a progressive destruction of articular and periarticular structure.[2] Inflammatory mediators generally produce joint inflammatory characteristics and permanent deformity with time if remain untreated.[3] RA is characterized by the synovitis of small and large joints, which may cause destruction of cartilage and bones leading to significant disabilities due to erosion of bones surface, if the condition is untreated.[4] Polymorphonuclear leukocytes and macrophages are stimulated, which results in the production of inflammation mediators including an important amount of superoxide and hydrogen peroxide.[5,6] Free radicals’ production is important for normal metabolism, but they can be destructive if their toxicity is not controlled by intracellular antioxidant defense mechanisms.[7] The study of antioxidant status during a free radical challenge can be used as an index of protection against the development of degenerative inflammatory processes in experimental conditions for therapeutic procedures.[7]
RA is treated with analgesic medications. However, some analgesics have been withdrawn from the market due to adverse drug reactions.[8] Research showed that many nonsteroidal anti-inflammatory drugs are known to cause gastric ulceration. Thus, natural products in general and medicinal plants are believed to be an important source of new chemical substances with potential therapeutic efficacy.[9]
Allanblackia gabonensis belongs to the family Guttiferae and commonly grows in tropical Africa (Cameroon, Gabon, Democratic Republic of Congo, etc.) at around 500 and 1750 m of altitude.[10] A. gabonensis is largely used in traditional medicine to improve virility in men and to treat infections such as dysenteries, cold, and toothaches[11,12] Population also uses A. gabonensis to relieve pain, inflammation, and rheumatism. The previous reports showed its antimicrobial, antileishmanial,[13] and analgesic and anti-inflammatory properties.[14] Phytochemical studies showed that the stem bark of A. gabonensis contains phytosterols, xanthone derivatives, epicatechin, and polyphenol [Figure 1].[14] Xanthone, benzophenone, flavonoid, and phytosterol were isolated as well.[13] Following a successive chromatography of the MeOH extract of the fruits of A. gabonensis over silica gel and Sephadex LH-20 done by Nganou et al,[15] there was isolation of a new polyprenylated benzophenone (1) along with the known kaempferol (2), morelloflavone (3), morelloflavone 7’-O-β-dglucopyranoside (4), β-sitosterol 3-O-β-d-glucopyranoside, and β-sitosterol [Figure 2]. The purpose of this study was to investigate the anti-inflammatory and antioxidant effects of A. gabonensis stem bark extract in rats.
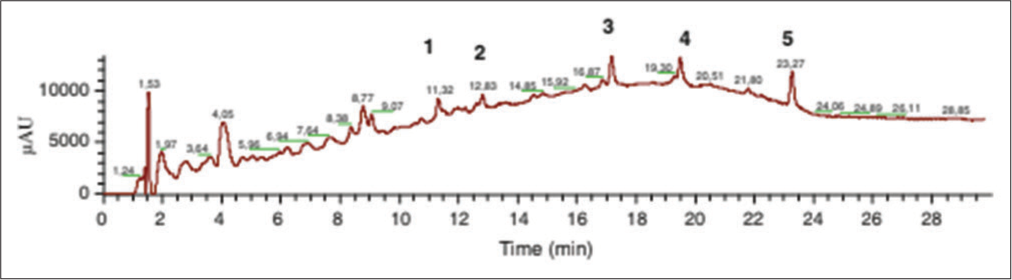
- Fingerprint of Allanblackia gabonensis, epicatechin (1), polyphenol derivatives (2, 3), xanthone derivatives (4), and phytosterol and others (5).
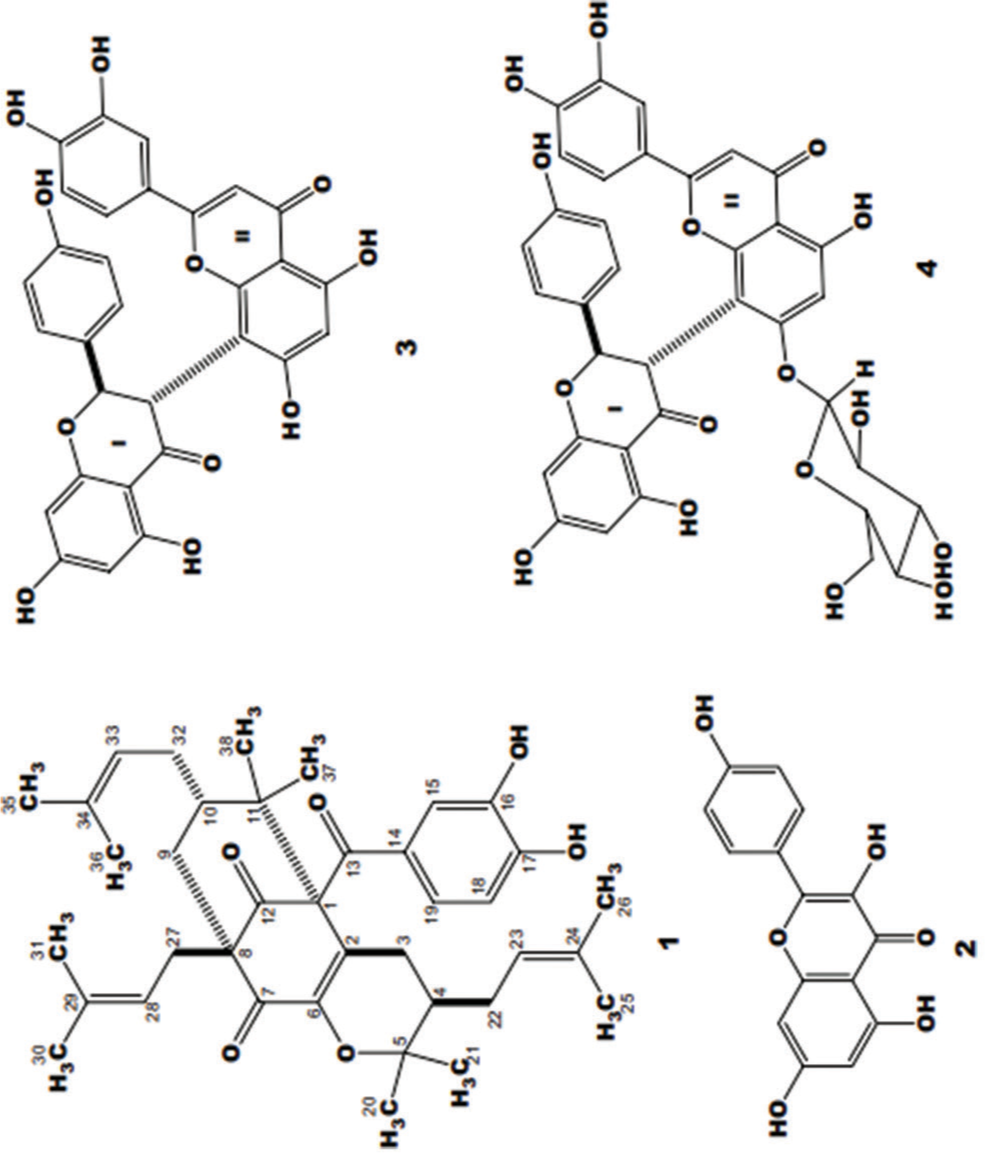
- Chemical structures from MeOH extract of the fruits of Allanblackia gabonensis.
MATERIAL AND METHODS
Plant material
A. gabonensis stem bark was collected in June 2007 on the Kola Mountain at Nkolbisson, Yaoundé/Cameroon and identified at the National Herbarium of Cameroon where a voucher specimen N° 23255/SRF/Cam was deposited for future reference. Pictures of stem and leaves were taken in 2012 at Kola Mountain at Nkolbisson [Figure 3].

-
Allanblackia gabonensis (a) leaves, (b) stem, (c) flowers, and (d) seeds.
Preparation of extract and phytochemistry studies
The air-dried stem bark was powdered, and the extraction was done by a decoction of 1 kg in distilled water (3 L) for 15 min. After filtration, the filtrate was concentrated in the oven at 55°C giving a brown residue of 83 g (that is 8.3% yield). High-performance liquid chromatography confirmed the presence of phytosterols (antioxidant activities[16]), xanthone (derivatives), epicatechin (antioxidant activities[17]), and polyphenol derivatives.[14]
Animals
Albino rats of both sexes, 3 months old and weighing 120–150 g, were used in this study. All the animals were bred in the animal house of the Department of Animal Biology and Physiology, University of Yaoundé I, Cameroon. Animals were housed in cages under standard laboratory conditions (12:12 light/dark cycle at 25°C ± 2°C) with free access to water and a standard commercial diet.
Experimental design
Rats were divided into five groups of six animals each, and fasted 12 h before the experiment. Induction of arthritis was done by the administration of 0.1 mL of CFA intradermically into the right hind footpad of each rat.
Group 1 served as the normal control, while Groups 2 and 3 received gavage distilled water (negative control) and dexamethasone (DEXA) starting on day 7 of the experiment (positive control), respectively. Groups 4 and 5 were treated by gavage with plant extract at the doses of 100 and 200 mg/kg, respectively, starting on day 7 of the experiment. On day 22 of the experiment, animals were fasted for 12 h and, thereafter, sacrificed by decapitation. For each animal, one aliquot of blood sample was collected with ethylenediaminetetraacetic acid (EDTA) for hematological index, and another aliquot was collected into the dry tube. The latter aliquot was centrifuged after 2 h; the serum was collected and used for biochemical parameters. The liver, kidney, spleen, and heart were dissected out of animals and weighed. A 20% (weight/volume) homogenate of the liver, spleen, and kidney was prepared in a Tris-HCl (50 mM pH: 7.4) buffer, centrifuged and the supernatant used for biochemical analysis. The heart was homogenized in Mc Even solution (8.6 g of NaCl, 0.42 g of KCl, 0.109 g of NaH2PO4, 1 g of CO3HNa, 0.03 g of MgCl2, 2 g of glucose, all dissolved in 200 mL of distilled water, 0.28 g of CaCl2 dissolved, and added to the solution. The solution was then completed to 1 L).
Pharmacological activities
Anti-inflammatory activities of A. gabonensis aqueous extract
Throughout the treatment period, the anti-inflammatory activity was assessed by the measurement of the right hind paw volume of all arthritic animals before Freund’s complete adjuvant inoculation and then 3, 6, 9, 12, 15, 18, and 21 days after CFA injection. This paw edema was determined using a mercury plethysmometer.
Measurement of hematological and biochemical parameters
At the end of the experiment day 22, all animals were sacrificed by decapitation and the blood was collected, one part in tubes with (EDTA) for hematological tests and the second part centrifuged after 2 h for biochemical analysis. Transaminases and bilirubin were estimated using specific kits (Fortress Diagnostic, Antrim, United Kingdom), while total proteins and creatinine were determined using the procedure described by Gornall et al.[18] and Bartels and Bohmer,[19] respectively. Other biochemical markers such as cholesterol, superoxide dismutase (SOD), catalase (CAT), malondialdehyde (MDA), and nitric oxide (NO) were quantified as described in the literature,[20-22] reduced glutathione[23-25] was evaluated as well.
Statistical analysis
Data were expressed as mean ± standard error of the mean and analyzed with GraphPad Instat Software, comparisons between different groups were assessed by one-way Analysis of variance followed by the Dunnett’s test. P < 0.05 and P < 0.01 were regarded as statistically significant.
RESULTS
Effect of A. gabonensis aqueous extract on Freund’s complete adjuvant-induced arthritis paw thickness
[Figure 4] shows measurements of the paw edema of rats with CFA-induced arthritis which shows a decrease (P < 0.01) of inflammation in rats treated by DEXA (0.2 mg/kg of body weight) started on the 15th day. This decrease (P < 0.01) started on day 12 in rats treated with the plant extract at doses of 100 and 200 mg/kg. A. gabonensis significantly reduced inflammation on day 12 at both doses, showing a reduction of 61.76% and 74.05%, respectively, at 100 and 200 mg/kg. On day 15, DEXA caused a reduction of 128.89% compared to the CFA group. On day 18, DEXA (0.2 mg/kg) and extract at the dose of 200 mg/kg significantly reduced paw edema, by 104.17% and 63.33%, respectively. DEXA (0.2 mg/kg) and A. gabonensis at 100 and 200 mg/kg significantly reduced paw edema on day 21, respectively, at 264.29%, 72.88%, and 104% in comparison with CFA rats.

- Effect of Allanblackia gabonensis aqueous extract on Freund’s complete adjuvant-induced arthritis paw thickness (mL). Each bar represents mean ± standard error of the mean, n = 6. CFA: Rats treated with adjuvant, CFA + Dexamethasone (DEXA): Rats treated with adjuvant and DEXA, CFA + E 100: Rats treated with adjuvant and extract at the dose of 100 mg/kg, CFA + E 200: Rats treated with adjuvant and extract at the dose of 200 mg/kg ##P < 0.01 compared to CFA group.
Effect of A. gabonensis aqueous extract on relative organ weight
As noted in [Figure 5], no significant difference was observed in relative weight among all groups.
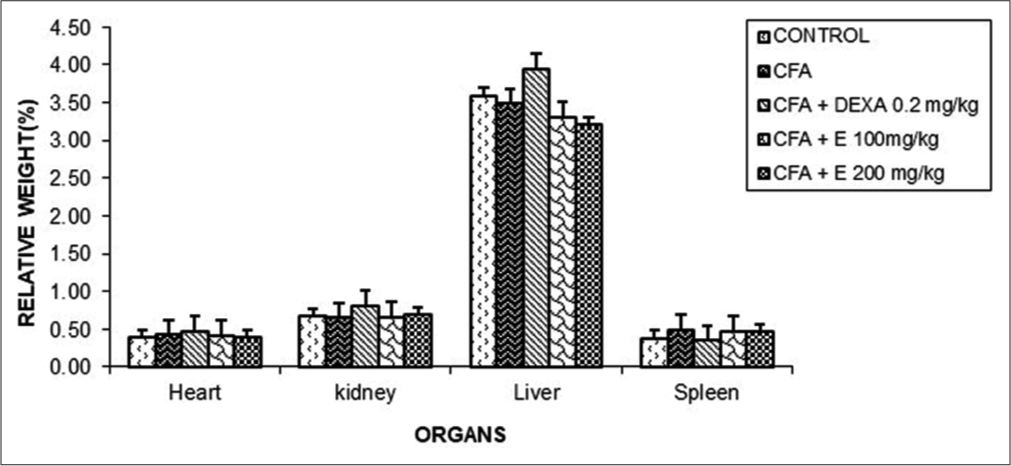
- Effect of Allanblackia gabonensis aqueous extract on relative organ weight. Each bar represents the mean ± standard error of the mean, n = 6. Control: Rats without any treatment, CFA: Rats treated with adjuvant, CFA + Dexamethasone (DEXA): Rats treated with adjuvant and DEXA, CFA + E 100: Rats treated with adjuvant and extract at the dose of 100 mg/kg, CFA + E 200: Rats treated with adjuvant and extract at the dose of 200 mg/kg.
Effect of A. gabonensis aqueous extract on blood cells
From [Figure 6], a significant increase in white corpuscles was observed in CFA group compared to control rats. Red blood cells and platelets did not significantly change during the experiment. No significant change was observed in platelets as compared to control.
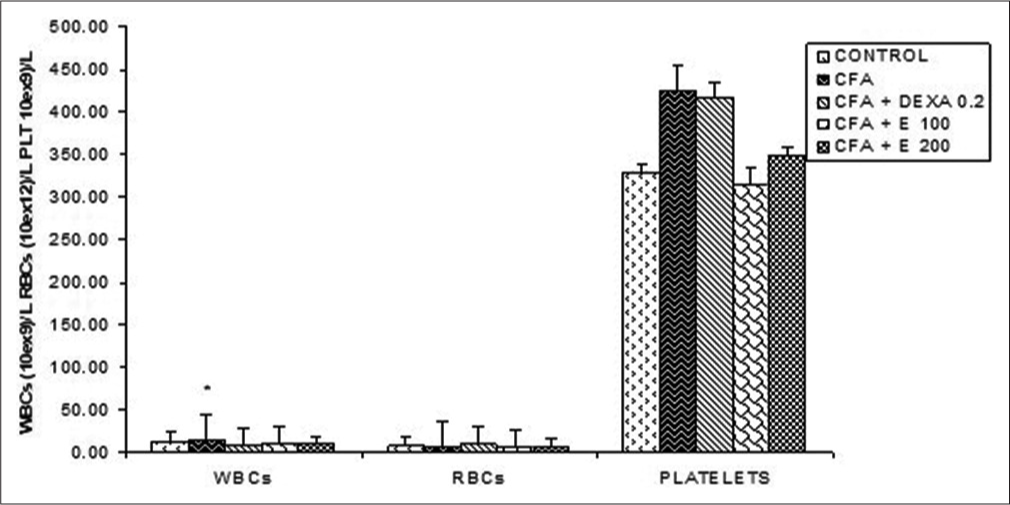
- Effect of Allanblackia gabonensis aqueous extract on blood cells. Each bar represents the mean ± standard error of the mean, n = 6. Control: Rats without any treatment, CFA: Rats treated with adjuvant, CFA + Dexamethasone (DEXA): Rats treated with adjuvant and DEXA, CFA + E 100: Rats treated with adjuvant and extract at the dose of 100 mg/kg, CFA + E 200: Rats treated with adjuvant and extract at the dose of 200 mg/kg *P < 0.05, compared to the control group without any treatment.
Effect of A. gabonensis aqueous extract on transaminase levels
[Figure 7] presents a significant increase (P < 0.01) of serum transaminase activity in the CFA group and rats treated with plant extract at the dose of 100 mg/kg were observed. A. gabonensis extract at the dose of 200 mg/kg significantly reduced serum AST compared to CFA rats, the percentage of reduction was 29.16%. DEXA (0.2 mg/kg) significantly reduced AST activities at a percentage of 77.74% as compared to the CFA group.
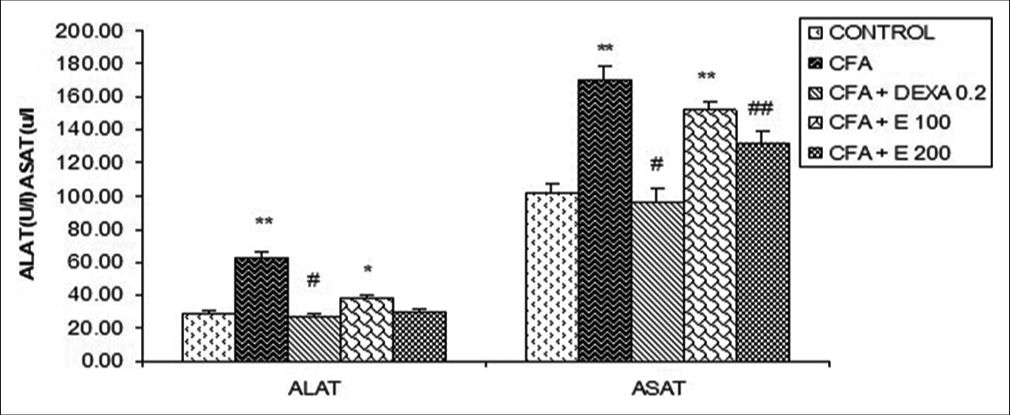
- Effect of Allanblackia gabonensis aqueous extract on transaminase levels. Each bar represents the mean ± standard error of the mean, n = 6. Control: Rats without any treatment, CFA: Rats treated with adjuvant, CFA + Dexamethasone (DEXA): Rats treated with adjuvant and DEXA, CFA + E 100: Rats treated with adjuvant and extract at the dose of 100 mg/kg, CFA + E 200: Rats treated with adjuvant and extract at the dose of 200 mg/kg *P < 0.05, **P < 0.01 compared to the control group without any treatment, #P < 0.05, ##P < 0.01 compared to CFA group.
Effect of A. gabonensis aqueous extract on creatinine levels
[Figure 8] shows that creatinine levels in the serum of the CFA group were significantly increased in comparison with the control group. The extract at 100 and 200 mg/kg significantly reduced creatinine levels in the kidney when compared to the control and CFA groups. DEXA has significantly reduced creatinine levels when compared to the CFA group, the percentage of reduction was 116.6%. There were no significant changes in kidney creatinine.
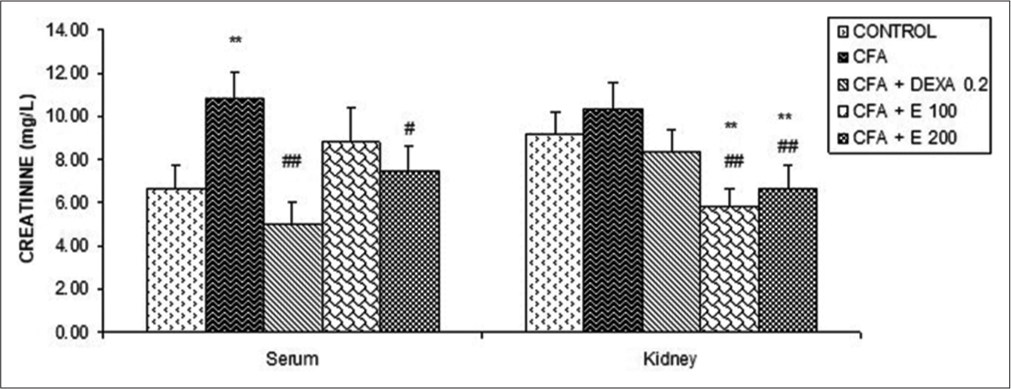
- Effect of Allanblackia gabonensis aqueous extract on creatinine levels. Each bar represents the mean ± standard error of the mean, n = 6. Control: Rats without any treatment, CFA: Rats treated with adjuvant, CFA + Dexamethasone (DEXA): Rats treated with adjuvant and DEXA, CFA + E 100: Rats treated with adjuvant and extract at the dose of 100 mg/kg, CFA + E 200: Rats treated with adjuvant and extract at the dose of 200 mg/kg **P < 0.01 compared to the control group without any treatment. #P < 0.05, ##P < 0.01 compared to CFA group.
Effect of A. gabonensis aqueous extract on bilirubin levels
[Figure 9] shows that a significant increase (P < 0.01) in the bilirubin rate was noted in CFA animals compared to the control group. Bilirubin levels were significantly reduced (P < 0.05) in rats receiving A. gabonensis at the doses of 100 and 200 mg/kg compared to the control group. A significant reduction (P < 0.01) was observed in animals that received DEXA (0.2 mg/kg), describing a reduction of 74.36% in comparison with the CFA group.
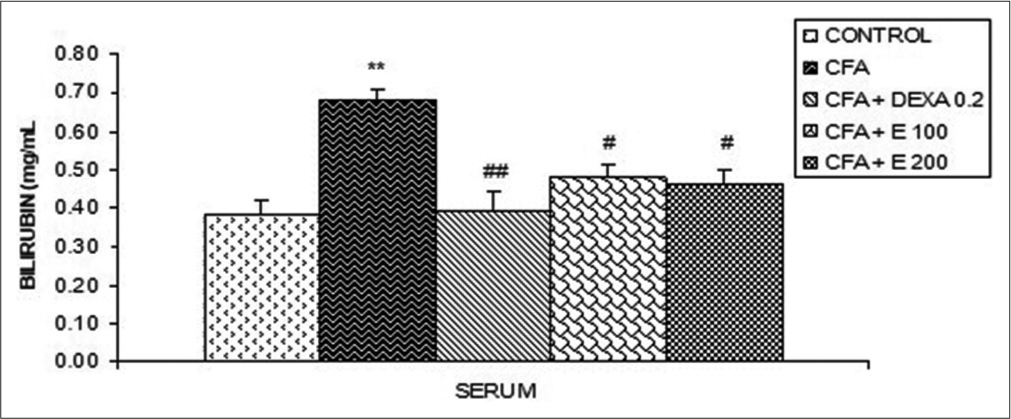
- Effect of Allanblackia gabonensis aqueous extract on bilirubin levels. Each bar represents the mean ± standard error of the mean, n = 6. Control: Rats without any treatment, CFA: Rats treated with adjuvant, CFA+ Dexamethasone (DEXA): Rats treated with adjuvant and DEXA, CFA + E 100: Rats treated with adjuvant and extract at the dose of 100 mg/kg, CFA + E 200: Rats treated with adjuvant and extract at the dose of 200 mg/kg **P < 0.01 compared to the control group without any treatment. #P < 0.05, ##P < 0.01 compared to CFA group.
Effect of A. gabonensis aqueous extract on total cholesterol
[Figure 10] shows that no significant differences were observed in total cholesterol as compared to the control and CFA groups. An increase of 22.5% was observed in the CFA group when compared to control. In DEXA and CFA + extract at 200 mg/kg, the increase was 20.5% and 20.8%, respectively, when compared to the control group.
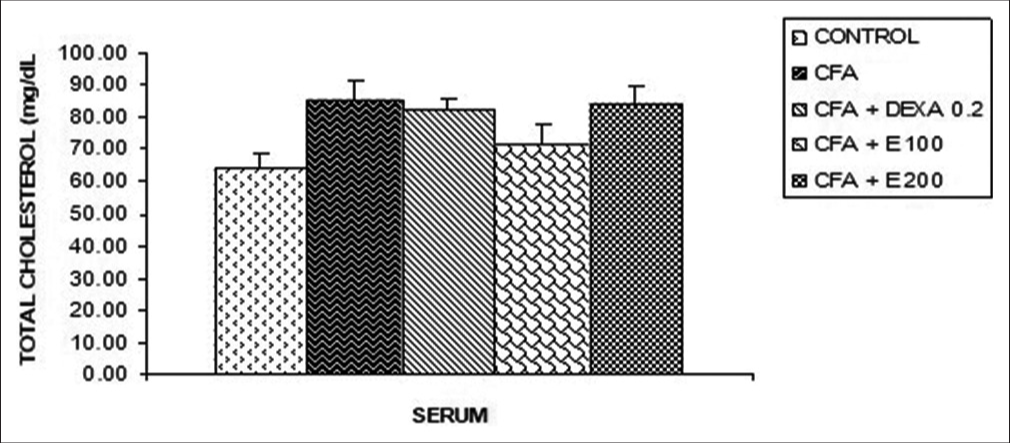
- Effect of Allanblackia gabonensis aqueous extract on total cholesterol. Each bar represents mean ± standard error of the mean, n = 6. Control: Rats without any treatment, CFA: Rats treated with CFA, CFA + Dexamethasone (DEXA): Rats treated with CFA and DEXA, CFA + E 100: Rats treated with CFA and extract at the dose of 100 mg/kg, CFA + E 200: Rats treated with CFA and extract at the dose of 200 mg/kg.
Effect of A. gabonensis aqueous extract on MDA levels
From [Figure 11], a significant (P < 0.01) increase in MDA levels was observed in the liver and heart of the CFA group compared to the control animals. A. gabonensis significantly reduced MDA activity in the liver and kidney at both doses, describing a reduction of 139.87% at 100 mg/kg and 160.91% at 200 mg/kg in the liver. The reduction rate was 109.09% at 100 mg/kg, and 86.49% at 200 mg/kg in the kidney.

- Effect of Allanblackia gabonensis aqueous extract on Malondialdehyde levels. Each bar represents mean ± standard error of the mean, n = 6. Control: Rats without any treatment, CFA: Rats treated with adjuvant, CFA + Dexamethasone (DEXA): Rats treated with adjuvant and DEXA, CFA + E 100: Rats treated with adjuvant and extract at the dose of 100 mg/kg, CFA + E 200: Rats treated with adjuvant and extract at the dose of 200 mg/kg *P < 0.05, **P < 0.01 compared to the control group without any treatment, ##P < 0.01 compared to CFA group.
Effect of A. gabonensis aqueous extract on CAT levels
From [Figure 12] a significant decrease of CAT was observed in the CFA group in the kidney and spleen compared to the control group. A significant increase (P < 0.01) of liver and cardiac CAT was observed in animals receiving extract at 100 and 200 mg/kg in comparison with the control group and CFA group. In the kidney, A. gabonensis at 200 mg/kg and indomethacin (0.2 mg/kg) significantly increased CAT levels when compared to CFA rats.

- Effect of Allanblackia gabonensis aqueous extract on catalase activity. Each bar represents mean ± standard error of the mean, n = 6. Control: Rats without any treatment, CFA: Rats treated with adjuvant, CFA + Dexamethasone (DEXA): Rats treated with adjuvant and DEXA, CFA + E 100: Rats treated with adjuvant and extract at the dose of 100 mg/kg, CFA + E 200: Rats treated with adjuvant and extract at the dose of 200 mg/kg **P < 0.01 compared to the control group without any treatment, ##P < 0.01 compared to CFA group.
Effect of A. gabonensis aqueous extract on glutathione levels
[Figure 13] shows that a significant decrease (P < 0.05) of glutathione was observed in the serum, liver, heart, and spleen in CFA group compared to the control. These reductions were 22.2%, 41.67%, and 36.92%, respectively. No significant modifications in the glutathione levels were observed in the kidney. A. gabonensis at the doses of 100 and 200 mg/kg significantly increased glutathione levels in the liver, describing the percentages of 33.33% and 30.5%, respectively. Only the dose of 200 mg/kg of the extract significantly increased (36.36%) this level in the spleen in comparison with the CFA group. It was observed a significant increase of glutathione in the serum and the organs in rats that received DEXA at 0.2 mg/kg compared to CFA group.
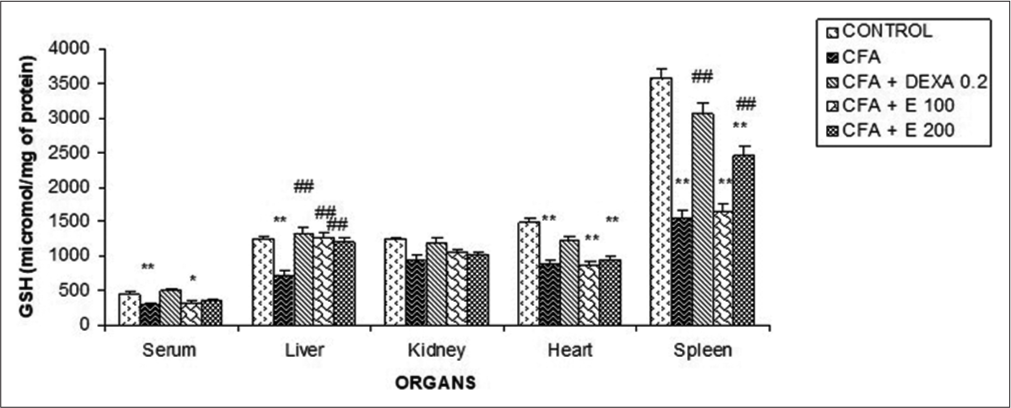
- Effect of Allanblackia gabonensis aqueous extract on glutathione activity. Each bar represents the mean ± standard error of the mean, n = 6. Control: Rats without any treatment, CFA: Rats treated with adjuvant, CFA + Dexamethasone (DEXA): Rats treated with adjuvant and DEXA, CFA + E 100: Rats treated with adjuvant and extract at the dose of 100 mg/kg, CFA + E 200: Rats treated with adjuvant and extract at the dose of 200 mg/kg *P < 0.05, **P < 0.01 compared to the control group without any treatment, ##P < 0.01 compared to CFA group.
Effect of A. gabonensis aqueous extract on NO levels
From [Figure 14] a significant increase (P < 0.01) of NO levels was observed in all the explored organs and serum of animals treated with CFA when compared to control. No significant changes in NO levels were observed in animal-received plant extract when compared to control. However, a significant decrease was observed when compared to the CFA group. A. gabonensis significantly reduced NO levels in the serum, kidney, heart, and spleen in comparison with the CFA group. The reduction in rats that received extract at the dose of 200 mg/kg was 69.13% in the kidney and 46.74% in the liver compared to the CFA group. This reduction was 47.39% for rats that received DEXA compared to the CFA group. In kidney, the reduction percentage was 48.67% in DEXA rats.
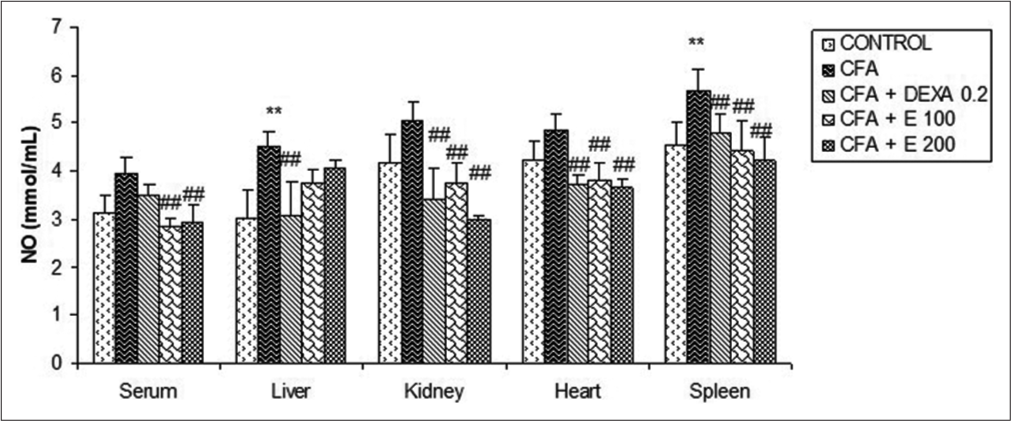
- Effect of Allanblackia gabonensis aqueous extract on nitric oxide levels. Each bar represents mean ± standard error of the mean, n = 6. Control: Rats without any treatment, CFA: Rats treated with adjuvant, CFA + Dexamethasone (DEXA): Rats treated with adjuvant and DEXA, CFA + E 100: Rats treated with adjuvant and extract at the dose of 100 mg/kg, CFA + E 200: Rats treated with adjuvant and extract at the dose of 200 mg/kg **P < 0.01 compared to the control group without any treatment, ##P < 0.01 compared to CFA group.
Effect of A. gabonensis aqueous extract on SOD activity
[Figure 15] shows that the administration of aqueous extract did not cause any significant difference in the liver and heart. However, a significant decrease (P < 0.01) of SOD was observed in the kidney and spleen of all rats receiving products.
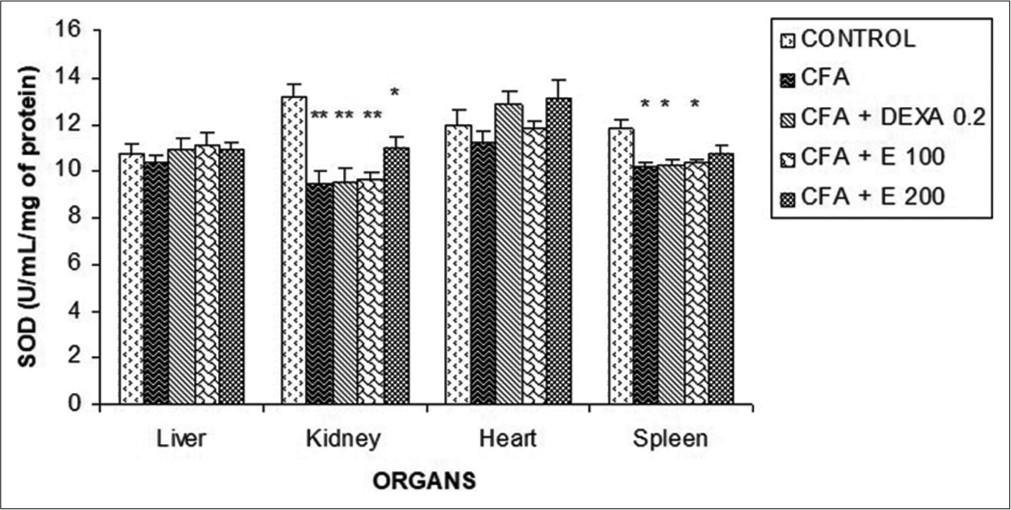
- Effect of Allanblackia gabonensis aqueous extract on superoxide dismutase activity. Each bar represents mean ± standard error of the mean, n = 6. Control: Rats without any treatment, CFA: Rats treated with adjuvant, CFA + dexamethasone (DEXA): Rats treated with adjuvant and DEXA, CFA + E 100: Rats treated with adjuvant and extract at the dose of 100 mg/kg, CFA + E 200: Rats treated with adjuvant and extract at the dose of 200 mg/kg *P < 0.05, **P < 0.01 compared to the control group without any treatment.
DISCUSSION
The present study was designed to evaluate the anti-inflammatory and antioxidant effects of A. gabonensis stem bark extract on Freund’s complete adjuvant (CFA)-induced arthritis in rats. CFA is generally used to evaluate inflammation at the chronic stage. The swelling was evident from day 3 to day 6 and started to decline on day 7 after adjuvant injection in rats receiving DEXA and plant extract at the dose of 200 mg/kg. The maximum reduction of inflammation was observed at day 12 in rats treated with plant extract at doses 100 and 200 mg/kg, and those receiving DEXA on day 15. These results might indicate that A. gabonensis extract exerts a pharmacological activity on chronic inflammation which may indicate its interference on the cyclo-oxygenase pathway.[26]
There is a mild to moderate rise in white blood cells (WBCs) count in arthritis conditions due to the release of interleukin (IL)-Iß inflammatory response. This IL-Iß increases the production of both granulocytes and macrophages colony-stimulating factors.[27,28] The increase of WBCs in the CFA group may be due to the persistence of inflammation in that group. The migration of leukocytes into inflamed areas of arthritis rats was partially suppressed by A. gabonensis aqueous extract and standard drug as reflected by the decrease in total WBCs, similar results were reported by.[29-31] Granulocytes which accumulate in rheumatoid joints are known to produce oxygen-derived free radicals during phagocytosis of bacteria and immune complexes.[7]
Transaminase activities were significantly increased in animals treated with CFA and those cotreated with CFA and 100 mg/kg plant extract. A. gabonensis at 200 mg/kg decreased the transaminase levels. No significant changes were observed in creatinine and bilirubin levels in animal-received extract.
There is a correlation between a reduction of NO and a decrease in inflammatory parameters.[32] NO production was decreased by the extract at both doses and this reduction may be attributed to a variety of mechanisms such as regulation of the production or activity of inducible nitric oxide synthase.[33]
Progress has been made regarding the source of the oxidative stress and an understanding has been achieved regarding the role of the signaling cascade that moderates the resulting inflammatory process.[34] Oxidants enhance IL-1, IL-8, and tumor necrosis factor production in response to inflammatory stimuli by activating the nuclear transcription factor, nuclear factor-kappa B. Sophisticated antioxidant defenses directly, and indirectly protect the host against the damaging influence of cytokines and oxidants.[35] SOD provided a potential defense against free radical damage. Some significant decreases of SOD were observed in the kidney and spleen comparatively to the control, this decrease might be attributed to the reduction in the lipid peroxidation and free radical reaction by the drugs.[7] Glutathione and CAT play a capital role in the detoxification of superoxide anion H2O2, thereby protecting the cells against oxidative free radicals.[36] CFA decreased the activities of SOD in the kidney and spleen. Lipid peroxides are formed by auto-oxidation reactions of polyunsaturated fatty acids found primarily in cell membranes.[37] Oxygen-derived free radicals during phagocytosis of bacteria and immune complexes, which may lead to the increase of lipid peroxide formation, are produced by granulocytes, which accumulate in the rheumatoid joints.[38] The extent of lipid peroxidation which is measured through MDA activity, a pro-oxidant factor that determines the oxidative damage[39] was found to be increased in CFA group compared to the normal one, this suggests that the tissues were exposed to an increase of oxidative stress. It was demonstrated that CFA significantly increased oxidative stress by decreasing the levels of SOD and CAT causing an increase in transaminase and alkaline phosphatase as well.[40] A. gabonensis caused the decrease of MDA in all the analyzed organs in animals that received extracts and DEXA. This decrease might be attributed to the presence of antioxidant components in the extract. The fact that aqueous extract of A. gabonensis did not affect spleen weight in CFA-induced arthritis in rats could explain the fact that the anti-inflammation effect of A. gabonensis was not directly related to specific immune responses. In this study, the dysfunction in the antioxidant system is indicated by the decrease in SOD and CAT activities and the increase of MDA levels in the CFA group compared to the control.
CONCLUSION
Anti-inflammatory activities coupled with the antioxidant properties of the aqueous stem bark extract of A. gabonensis in Freund’s complete adjuvant-induced arthritis in rats appears to be really promising as arthritis medication. Further studies are extended to elucidate the mechanism(s) of action, responsible for the observed antioxidant and anti-arthritic activities. Moreover, more detailed preclinical and clinical studies especially in humans are highly recommended.
Acknowledgments
We thank Prof. Faustin Pascal Tsafack Manfo (University of Buea in Cameroon) for his support in editing this. We also thank Prof. Paulin Nana (University of Ebolowa in Cameroon) for his help in laboratory materials as well as his assistance in getting the experiment done.
Ethical approval
The author(s) declare that the experiment was conducted in accordance with an institutional protocol approved by the National Ethical Committee (Reg. no. FWAIRD 0001954).
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- T lymphocyte lines induce autoimmune encephalomyelitis (EAE), delayed hypersensitivity and bystander encephalitis or arthritis. Eur J Immunol. 1984;14:729-34.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of red and white seed extracts of Abrus precatorius Linn against freund's complete adjuvant induced arthritis in rats. J Med Plants Res. 2007;19:86-94.
- [Google Scholar]
- Evaluation of anti-arthritic potential of Hybanthus eneaspermus. Afr J Pharm Pharmacol. 2009;3:611-4.
- [Google Scholar]
- Rheumatoid arthritis: What have we learned about the causing factors? Pak J Pharm Sci. 2016;29:629-45.
- [Google Scholar]
- Macrophages and inflammatory mediators in tissues injury. Annu Rev Pharmacol Toxicol. 1995;35:655-77.
- [CrossRef] [PubMed] [Google Scholar]
- Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 2003;54:469-87.
- [Google Scholar]
- Salubrious effect of Semecarpus anacardium against lipid peroxidative changes in adjuvant arthritis studied in rats. Mol Cell Biochem. 1997;175:65-9.
- [CrossRef] [PubMed] [Google Scholar]
- Post-marketing withdrawal of analgesic medications because of adverse drug reactions: A systematic review. Expert Opin Drug Saf. 2018;17:63-72.
- [CrossRef] [PubMed] [Google Scholar]
- Analgesic and anti-inflammatory activities of Buchanania lanzan Spreg. Roots Res J Pharm Biol Chem Sci. 2011;2:419-25.
- [Google Scholar]
- Flore du Congo, Rwanda et Burundi, Spermatophytes Guttiferae. Bruxelles, Jardin Botanique National de Belgique 1970:40.
- [CrossRef] [Google Scholar]
- Fruitiers sauvages d'afrique In: Espèce du Cameroun. Wageningen: CTA; 1996. p. :1-416.
- [Google Scholar]
- Antimicrobial and Antileishmanial xanthones from the stem bark of Allanblackia gabonensis (Guttiferae) Nat Prod Res. 2008;22:333-41.
- [CrossRef] [PubMed] [Google Scholar]
- Analgesic and anti-inflammatory effect of aqueous extract of the stem bark of Allanblackia gabonensis (Guttiferae) Inflammopharmacology. 2013;21:21-30.
- [CrossRef] [PubMed] [Google Scholar]
- Guttiferone BL with antibacterial activity from the fruits of Allanblackia gabonensis. Nat Prod Res. 2019;33:2638-46.
- [CrossRef] [PubMed] [Google Scholar]
- Antioxidant effects of phytosterol and its components. J Nutr Sci Vitaminol (Tokyo). 2003;49:277-80.
- [CrossRef] [PubMed] [Google Scholar]
- (-)-Epicatechin-an important contributor to the antioxidant activity of Japanese knotweed rhizome bark extract as determined by antioxidant activity-guided fractionation. Antioxidants (Basel). 2021;10:133.
- [CrossRef] [PubMed] [Google Scholar]
- Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949;177:751-66.
- [CrossRef] [PubMed] [Google Scholar]
- Estimation of creatinine clearance in patient with unstable renal function. Clin Chim Acta. 1972;37:193-7.
- [CrossRef] [PubMed] [Google Scholar]
- The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170-5.
- [CrossRef] [Google Scholar]
- Remarks on the treatise by H.H. Weselsky and Benedikt. Purified azo compounds. Chem Ber. 1879;12:426-8.
- [CrossRef] [Google Scholar]
- Anti-inflammatory effect of Spirulina fusiformis on adjuvant-induced arthritis in mice. Biol Pharm Bull. 2006;29:2483-7.
- [CrossRef] [PubMed] [Google Scholar]
- Rheumatoid arthritis and its therapy In: The texbook of therapeutic drugs and disease management (6th ed). Baltimore: Williams and Wilkins Company; 1996. p. :579-95.
- [Google Scholar]
- Arthritis and allied conditions In: A textbook of rheumatology (15th ed). Tokyo: A Waverly Company; 2001. p. :207-26.
- [Google Scholar]
- Influence of ethanolic extract of Borassus flabellifer L. male flowers (inflorescences) on chemically induced acute-inflammation and poly arthritis in rats. Pharm Technol. 2008;1:551-6.
- [Google Scholar]
- Anti-arthritic activity of Premna serratifolia Linn, wood against adjuvant induced arthritis. Avicenna J Med Biotechnol. 2010;2:101-6.
- [Google Scholar]
- Therapeutic effect of a poly-herbal preparation on adjuvant induced arthritis in Wistar rats. Int J Pharm Pharm Sci. 2011;3:186-92.
- [Google Scholar]
- Comparison of the nitric oxide synthase inhibitors methylarginine and aminoguanidine as prophylactic and therapeutic agents in rat adjuvant arthritis. J Rheumatol. 1995;22:1922-8.
- [Google Scholar]
- Mitochondrial DNA polymorphisms in bipolar disorder. J Affect Disord. 2002;62:151-64.
- [CrossRef] [PubMed] [Google Scholar]
- Oxidative stress and inflammation in heart disease: Do antioxidants have a role in treatment and/or prevention? Int J Inflam. 2011;2011:514623.
- [CrossRef] [PubMed] [Google Scholar]
- Nutritional antioxidants and the modulation of inflammation: Theory and practice. New Horiz. 1994;2:175-85.
- [Google Scholar]
- Tissu lipidation and antioxidant status in patients with aenocarcinoma of the breast. Clin Chim Acta. 2002;325:165-70.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of anti-inflammatory activity of Cleome gynandra L. Leaf extract on acute and chronic inflammatory arthritis studied in rats. J Pharmacol Toxicol. 2007;2:44-53.
- [CrossRef] [Google Scholar]
- Impaired function of polymorphonuclear neutrophilic granulocytes in rheumatoid arthritis. Immun Infekt. 1993;21(Suppl 1):15-6.
- [Google Scholar]
- Antioxidant activity of arthritin--a polyherbal formulation. Indian J Exp Biol. 2006;44:403-7.
- [Google Scholar]
- Sarcococca saligna hydroalcoholic extract ameliorates arthritis in complete freund's adjuvant-induced arthritic rats via modulation of inflammatory biomarkers and suppression of oxidative stress markers. ACS Omega. 2022;7:13164-77.
- [CrossRef] [PubMed] [Google Scholar]







