Translate this page into:
Deranged hembiosynthetic pathway in gasoline dispensers in Nigeria: Implications for risk of myeloproliferative disorders and chemoprevention

-
Received: ,
Accepted: ,
How to cite this article: Anetor JI, Adigun TO, Bolajoko EB, Anetor GO, Orimadegun BE, Akiibinu MO, et al. Deranged haembiosynthetic pathway in gasoline dispensers in Nigeria: Implications for risk of myeloproliferative disorders and chemoprevention. Am J Biopharm Pharm Sci 2022;2:2.
Abstract
Objectives:
There is increasing exposure to petrochemicals, including benzene, particularly in the low and medium-income countries. Benzene is a component of many petrochemicals and a ubiquitous environmental pollutant. Phenol is one of its principal metabolites and serves as a biomarker of exposure to benzene. The mechanism of its toxicity is incompletely elucidated. Benzene’s interaction with key micronutrients; copper (Cu), iron (Fe), and zinc (Zn) in the haemopoietic system has only been poorly explored, particularly in the developing countries where their status is variable and uncertain, with attendant intense exposure to petrochemicals.
Material and Methods:
Two groups of 50 gasoline dispensers (GDs) and 50 non-occupationally exposed participants were selected from Oye Local Government Area, Nigeria. The duration of occupational exposure was 2–10 years. Serum levels of Cu, Fe, and Zn were determined using flame atomic absorption spectrophotometry while heme and phenol were determined by standard spectrophotometry.
Results:
Phenol was significantly higher in GDs (P = 0.000), compared to controls (P < 0.05). The micronutrients, Cu, Fe, and Zn were all significantly decreased in GDs compared to controls (P = 0.000 in all cases). Phenol and Fe demonstrated significant inverse correlation (r = −0.557, P = 0.00), while heme and Zn also exhibited inverse correlation respectively to phenol (r = −0.38, P = 0.01; r = −0.37, P = 0.01).
Conclusion:
These data suggest intense perturbation of the haemopoietic system in GDs; likely from altered xenobiotic metabolism requiring heme in cytochrome P450; cell cycle dysregulation, where Zn is pivotal, p53 suppression also dependent on Zn and oxidative stress all converging in haemopoietic dysregulation. Importantly, depression of these micronutrients implies potentiation of myelotoxicity and risk of myeloproliferation, probably arising from alterations in transcription, differentiation errors, genome instability, and derangement in cell signal transduction moderated by Zn; accentuating risk of myeloproliferation; suggesting a role for these micronutrients in chemoprevention. Understanding these events may be important in risk assessment, policy formulation, regulatory measures and chemoprevention in GDs and the general population.
Keywords
Benzene chemoprevention
Gasoline dispensers
Micronutrients
Myeloproliferative syndrome
INTRODUCTION
Growing evidence indicates increasing exposure to petrochemicals, including benzene, particularly in the developing countries. [1-3] Benzene is a major component of gasoline to which gasoline dispensers (GDs) are persistently exposed in the occupational environment. This exposure may be additional to that from the general environment, alluding to the observation that the world today may be considered a chemical habitat with calls for improved understanding.[4] Benzene is a volatile aromatic hydrocarbon and a component of crude oil and gasoline; that is also the starting point of many synthetic processes in the chemical industries. The current interest of the scientific community and lay public about environmental chemicals may be a resurgence of earlier calls by Rachel Carson nearly six decades ago and later called to question by Trewavas et al.[5] Although the focus at the time was on pesticides, currently one of the most prevalent chemicals, particularly in the developing countries may be considered to be benzene.[1-3] Benzene being one of the numerous chemicals in gasoline and related products is ubiquitous.[6,7] and has been consistently linked to leukemia,[8] hemoproliferative disorder characterized by the presence of malignant haemopoietic cells within the peripheral circulation or bone marrow.
The understanding of the molecular mechanisms by which benzene dysregulates hematopoiesis, precipitating leukemia though significantly advanced is incompletely elucidated.[9] Hunter in his early observation associated benzene with acute myeloid leukemia.[10] His observation was however largely ignored until more recent advances were made, clearly connecting exposure to benzene and related agents with blood dyscrasias, including acute myeloid leukemia.[10]
In the developed countries, benzene was used on a large scale as an organic solvent, but its severe toxicity led to its restricted general use at a concentration of 1% or less in any solvent.[11] In addition, it is used entirely in closed systems in these countries so that exposures are limited in essential processes.[9] In contrast, in the developing countries or Low- and Medium-Income Countries (LMICs) that are rapidly industrializing, such as Nigeria and other petroleum-exporting countries, the use of benzene is at an unregulated large scale. It has been reported that the benzene content in gasoline in West Africa may be as high as 40%[12] and gasoline dispensing machines are dotted all over these countries without vapor recovery systems[3] or monitors. Environmental exposures, from locomotive and electricity generator emissions and cigarette smoke in the general population may also be contributory to the burden of benzene exposure.[13]
It has been reported that the ambient air around buildings at close proximity to petrol filling stations also have increased levels of benzene.[1-3] Thus, there is increased benzene exposure in the environment including residential areas but substantially accentuated by that from petrol stations.[1,2,14] Gasoline dispensing stations in Nigeria and other neighboring countries like Ghana are sited with little regard to regulations as the sale of petrol and related petrochemicals in these countries is a very big business and considered very lucrative,[3] thus employing a large number of workers. As recently observed by some investigators, exposure to benzene through gasoline dispensing is of great public health significance in both urban and rural areas in Nigeria.[3]
Epidemiological studies and case reports provide ample evidence of a causal relationship between occupational exposure to benzene and benzene-containing solvents and the occurrence of acute myelogenous leukemia.[15] Benzene, through its metabolites, including phenol and its downstream metabolites has been suggested to play central roles in the induction of leukemogenesis.[8,11] Hydroquinone a downstream metabolite of phenol is considered central in leukemogenesis associated with benzene exposure because of the hematotoxicity. These metabolites may cause DNA and chromosomal damage and mutation in key enzymes, leading to derangement of processes of cell growth and differentiation that may culminate in leukemia. Phenol also inhibits topoisomerase II (through its activation by myeloperoxidase and H2O2) consequently altering hematopoiesis and clonal selection.[8]
The possible role of the perturbation of the metabolism of key micronutrients, such as zinc (Zn), copper (Cu), and iron (Fe) in the hemebiosynthetic pathway in the pathogenesis of leukemia though previously suggested, [16,17] still leaves a lot of gaps to be explored. The precise cellular and molecular mechanisms remain areas of speculations. Imbalances in the optimal levels of some micronutrients have been linked with numerous adverse effects on biological processes and associated with cancer of other sites, as well as increased carcinogenic risk. Anetor et al.[18] reported, a high cadmiumZn ratio in cigarette smokers, providing evidence that the convergence of reduced Zn level, high cadmium: Zn ratio, increased Cu level and decreased total globulin may serve as a simple panel of biomarkers of risk of prostate cancer. Similar investigations involving benzene from an environment with peculiar characteristics that may raise susceptibility index are largely lacking. Repeated exposure to benzene may induce malignant transformation in the bone marrow and eventual disruption of the hematopoietic system. Bone marrow cells, the main sites of hematopoiesis, are particularly vulnerable to oxidative DNA and chromosomal damage, leading to derangement of cell growth mechanisms and differentiation that may be precursors to the evolution of leukemia.[19]
In addition, a growing number of studies and case reports have linked prolonged exposure to benzene to lymphopoietic cancers such as multiple myeloma and leukemia in refinery workers and others in related occupations.[15,20,21] Many of these studies have, however, not adequately examined the mechanisms and implications in occupational and environmental gasoline exposure in many LMICs including, Nigeria and approaches to mitigation. With the increased exposure to various sources of benzene in these highly polluted countries[1,3] there is increased risk of developing leukemia in both occupationally exposed workers and the general population. It is this great environmental and public health importance of petrochemical exposure that informed the investigation of a benzene metabolite and the risk of leukemia development in workers occupationally exposed to gasoline with implications for possible mitigation.
It is hoped that the study outcome may provide useful information for the scientific community to have a more in-depth assessment of the risk and advice on policy formulation, enabling regulatory measures in occupational and environmentally exposed populations to benzene in the LMICs and other countries where there is high exposure. It may also give rise to precautionary measures such as chemoprevention.
MATERIAL AND METHODS
Study site and study population
Fifty GDs of both genders (60% male and 40% female) with age range 18–65 years (Mean age; 24.6 ± 4.7) who gave their consent were recruited from the various gasoline stations located at the Oye Local Government Area (LGA) and environ, in Ekiti State, Nigeria. These participants had been occupationally exposed to gasoline for a minimum of 2 years. The total range of duration of occupational exposure was 2–10 years. Information was obtained through a detailed semi-structured pre-test questionnaire. Smokers were excluded from participating in the study, as this could be a confounding source of benzene. An educated guess based on previous reports considered sufficient to detect significant differences (power) when they exist was essentially adopted for arrival at the sample size. Equal number for GDs and control was enrolled.
Control population
Fifty non-occupationally exposed participants of both gender (60% males and 40% females) with age range 18–65 years (Mean age = 25.3 ± 4.8) were recruited from the same environment of Oye LGA and environ, Ekiti State, Nigeria. These participants were not occupationally exposed to gasoline and were not living in an environment with proximity to gasoline stations. Information was also obtained through a detailed semi-structured pre-test questionnaire as for the chemical workers.
Blood collection
Ten milliliters of venous blood were aseptically collected from the antecubital fossa vein with minimal stasis using pyrogen-free disposable needles and syringes. Five milliliters of the blood specimen were carefully dispensed into ethylenediaminetetraacetic acid vacutainer tubes and plain bottles. The blood samples in the plain bottles were allowed to clot to yield serum and then retracted before being centrifuged in a Centaur MSE centrifuge (Fisons, England) at 5000 revolution/min for 5 min. The blood sera were obtained and stored at −20°C until analysis. Urine samples (20 ml) were also collected from each participant (both cases and control) sterile universal bottles and preserved appropriately until analysis for determination of phenol level a biomarker (an indirect measure) of benzene exposure).
Determination of urinary phenol
Urinary phenol levels were determined according to the method developed by Lander et al.[22] a modification of the methods earlier described by Houghton and Pelly[23] and Hill and Herndon[24] using HPLC (Waters Model 616/626 LC system). Briefly, p-aminodimethylaniline and phenol in the presence of potassium reacted with ferricyanide to form indophenol.
Determination of micronutrients elements
The micronutrient elements, Cu, Fe, and Zn were determined with flame atomic absorption spectrophotometer (Buck 205 Model, Germany) at the Analytical Services Laboratory, International Institute of Tropical Agriculture, Ibadan, Nigeria. The principle and procedures adopted were based on the direct method as described by Kaneko.[25]
Determination of total heme by colorimetric method
Heme level was determined using the heme Assay kit which is based on the improved aqueous alkaline solution method, in which heme is converted into a uniform-colored complex. The intensity of the brownish-red color was measured at 400 nm and is directly proportional to the heme concentration in the sample. The optimized formulation substantially reduces interference by substances in raw samples and exhibits high sensitivity.[26]
Quality control
All analyses were subjected to rigorous quality control. Certified serum trace element samples (SAS Trace Element Centre, UKNEQAS for Trace Element Scheme) were used, and results were only accepted if they fell within mean ± 2 SD of the recommended values.
Statistical analysis
Data obtained from this study were subjected to statistical analysis using SPSS version 17.00 and Microsoft Office Excel; all values were expressed as mean ± standard error of mean (SEM) in the different study groups. Student’s t-test, Chi-square, and Pearson’s correlation were used to establish differences and relationships among variables, respectively. Analysis of variance was also carried out for the three groups of duration in occupational exposure. Results were considered significant at P < 0.05 and 95% confidence interval.
RESULTS
The exposed (GDs) had significantly lower educational status than the controls (which implies that most of the GDs had minimal formal education), but there was no significant difference in the percentage of male participants in comparison to that of the female subjects in each group [Table 1]. There was similarly no difference between the age of the participants exposed to gasoline compared to that of the unexposed group [Table 2].
| Parameter | GD (n=50) n% | Unexposed (n=50) n% | χ2 | P | ||
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 30 | 60 | 30 | 60 | 0.000 | 1.000 |
| Female | 20 | 40 | 20 | 40 | ||
| Educational level | ||||||
| Low | 41 | 82 | 25 | 50 | 11.284 | 0.001* |
| High | 9 | 18 | 25 | 50 | ||
| Parameter | Exposed subjects (n=50) | Unexposed subjects (n=50) | t | P |
|---|---|---|---|---|
| Age (yrs.) | 24.60±4.72 | 25.3±4.79 | −0.736 | 0.464 |
Strikingly, the levels of serum Cu, Fe, and Zn, as well as the level of heme were very significantly reduced in the workers exposed to gasoline (GDs) than in the unexposed (P < 0.001) in all cases [Table 3]. In contrast urinary phenol was significantly higher (over two-fold) in the chemical workers (GDs) than in the unexposed population (P < 0.001) [Table 3]. Significant positive correlation (r = 0.768, P < 0.001) was demonstrated between age and duration of occupational exposure in the chemical workers. Notably, heme was significantly negatively correlated with the toxic metabolite, phenol (r = −0.38, P < 0.007). Remarkably, phenol was also strongly inversely correlated with Zn (r = −0.37, P = 0.009) and inversely very strongly correlated with Fe (r = −0.55, P < 0.000). Although the correlation with Cu was also inverse, this was not significantly different (r = −0.19, P > 0.05). Fe, contrary to expectation did not correlate with heme (r = 0.28, P > 0.05).
| Parameters | Exposed (n=50) |
Unexposed subjects (n=50) | t | P |
|---|---|---|---|---|
| Fe (µg/dl) | 85.04±3.07 | 132.28±2.85 | 11.285 | 0.000* |
| Zn (µg/dl) | 55.89±1.49 | 100.52±2.27 | 16.423 | 0.000* |
| Cu (µg/dl) | 90.86±2.54 | 171.68±2.87 | 21.071 | 0.000* |
| Haem (µg/dl) |
72.94±2.85 | 142.43±6.87 | 9.349 | 0.000* |
| Phenol (µg/dl) |
276.19±18.02 | 120.49±5.94 | 8.207 | 0.000* |
Table 1 shows that lower educational level in GDs may put them at elevated risk of the deleterious effects of benzene from gasoline exposure.
Each value is mean ± SEM (n = 50). No significant difference in age between GDs and control (P > 0.05). Age of GDs and control is matched.
Table 3 shows that phenol level in the GDs is over two-fold that of controls, reflecting the intensity of exposure with attendant nearly 50% decrease in heme level compared to control. All the key trace elements in the heme biosynthetic pathway was significantly decreased (P<0.000), probably reflecting the severity of the adverse effects of exposure to gasoline.
Table 4 shows very strong inverse correlations probably reflecting the severity of effect. The strong inverse correlation of phenol to heme, Fe and Zn.
| Correlating pairs | Exposed participants (n=50) | |
|---|---|---|
| r | P | |
| Age vs. duration of occupational exposure | 0.768 | 0.000* |
| Duration occupational exposure vs. Phenol level | 0.107 | 0.460 |
| Age vs. Phenol level | 0.206 | 0.855 |
| Phenol/Fe | −0.553 | 0.000* |
| Phenol vs. Haem | −0.375 | 0.007* |
| Phenol vs. Cu | −0.019 | 0.898 |
| Phenol vs. Zn | −0.368 | 0.009* |
| Fe vs. Haem | 0.281 | 0.048* |
DISCUSSION
Benzene is a common environmental and industrial toxicant and an important component of gasoline. It is outstanding among the industrial poisons and has been consistently demonstrated to be an environmental pollutant that can induce hematotoxicity and haemopoietic neoplasia in both humans and animals.[8,27] Its toxicity has been demonstrated to involve both bone marrow depression and leukemogenesis caused by damage to multiple classes of hematopoietic stem cells (HSCs).[13,28,29] While some recent studies have demonstrated that certain cellular dysfunctional activities may occur, other reports have been variable, thus the understanding of the precise mechanisms still leaves a lot of gaps.[3,16] Altered erythrocyte-committed stem cells have largely been attributed to benzene metabolites in exposed populations.[30] The exact mechanism of the genesis of myeloproliferative disorders, of which leukemia is the most common form remains largely conjectural. The risk of development of leukemia in workers occupationally exposed to gasoline particularly in a developing tropical climate where benzene fumes may be higher than in temperate regions,[12] co-existent with sub-optimal nutritional status is a major feature of this. Data from this study have provided evidence addressing the preceding gaps and demonstrating the interactive roles of benzene metabolism with some key micronutrients in the hemebiosynthetic pathway and the likelihood of elevated risk of leukemia in workers exposed to gasoline.
The observed elevated urinary phenol level found in the occupationally exposed group is reflective of significant exposure to benzene by these GDs. Phenol is a key metabolite in the metabolism of benzene and serves as a precursor for other reactive metabolites of benzene, such as hydroquinone and 1, 4-benzoquinone [Figure 1]. The level of phenol in urine was employed as a biomarker of benzene exposure, as phenol is one of the principal metabolites of benzene that are freely excreted in the urine. This finding is consistent with an earlier report[9] in which substantially higher levels of urinary phenol were found in petrol dispensing attendants compared to individuals occupationally unexposed to gasoline. The high level observed probably corroborates the high content of benzene in gasoline consumed in West Africa.[12] Although the sensitivity of phenol as a biomarker of benzene exposure may be limited, in excessive exposure it serves as an acceptable biomarker of benzene exposure,[31] particularly where the benzene component of gasoline is very high.[12] Measurement of Urinary benzene is considered a better marker in subsequent studies may improve this. Kim et al.[31] found that the urine concentration of phenol was consistently elevated when the group’s median duration of benzene exposure was 2 years or over. Metabolite profile studies[32] have also demonstrated elevated levels of urinary metabolites in benzene-exposed workers.
This may, therefore, confirm that a high level of benzene exposure occurs via gasoline dispensing in this suburban community. Phenol is pivotal in the metabolism of benzene; it initiates the oxidation of hydroquinone (another metabolite of benzene) to the reactive metabolite 1, 4-benzoquinone by myeloperoxidase in the bone marrow in which the stem cells, myeloid progenitor cells and stromal cells are sensitive targets.[8,9,30] Reduction of the production of colony-stimulating factors by stromal fibroblasts may also be important.[8] The toxic effect of benzene metabolites on the bone marrow has been consistently suggested to be effected through pathways of benzene metabolism.[16] The bioactivated reactive metabolites cause growth factor dysregulation[16,33] oxidative stress and mutation.[8] DNA damage and impaired DNA repair,[17,18] as well as altered cell cycle regulation,[17] and inhibition of apoptosis.[17,18,33]
Thus benzene may exhibit multifactorial carcinogenicity.[34] Low et al.[35] have suggested that the carcinogenicity of benzene on target organs depends on the ability of enzymes in the organs to metabolize benzene, while the metabolism of benzene to reactive metabolites by hepatic enzymes such as cytochrome P450-2E1 (CYP2E1) is a prerequisite to cytotoxicity and genotoxicity associated with benzene exposure,[30] Benzene may through its bioactive metabolites also induce structural and numerical chromosome aberrations, sister chromatid exchanges and micronuclei formation through various routes of exposure.[8] Our results are consistent with previous reports.
The significantly reduced levels of Cu, Fe and Zn, in this study and their inverse correlation with urinary phenol are striking. This may in part be one of the important driving pathomechanisms of most of the biochemical and pathological derangements observed above. The cause of the reduced heme level may also at least in part be attributable to the observed aggregate reduced levels of the key heme pathway micronutrients; Cu, Fe, and Zn, culminating in reduced heme biosynthetic activity. This may have occurred because of the disruption of the integrated mechanism coordinating role ensuring that the right concentration of each micronutrient is in the right tissue or system.[36] Micronutrients including these trace elements play a number of key metabolic roles in the heme biosynthetic pathway. Alteration of Zn and Cu levels has been observed in lymphoproliferative disorders, among other tumors. Demir et al.[17] reported that the levels of Zn, along with those of Mg and Mn were lower while those of Cu, Pb, and Cd were higher in patients with acute myelogenous leukemia compared to a healthy group.
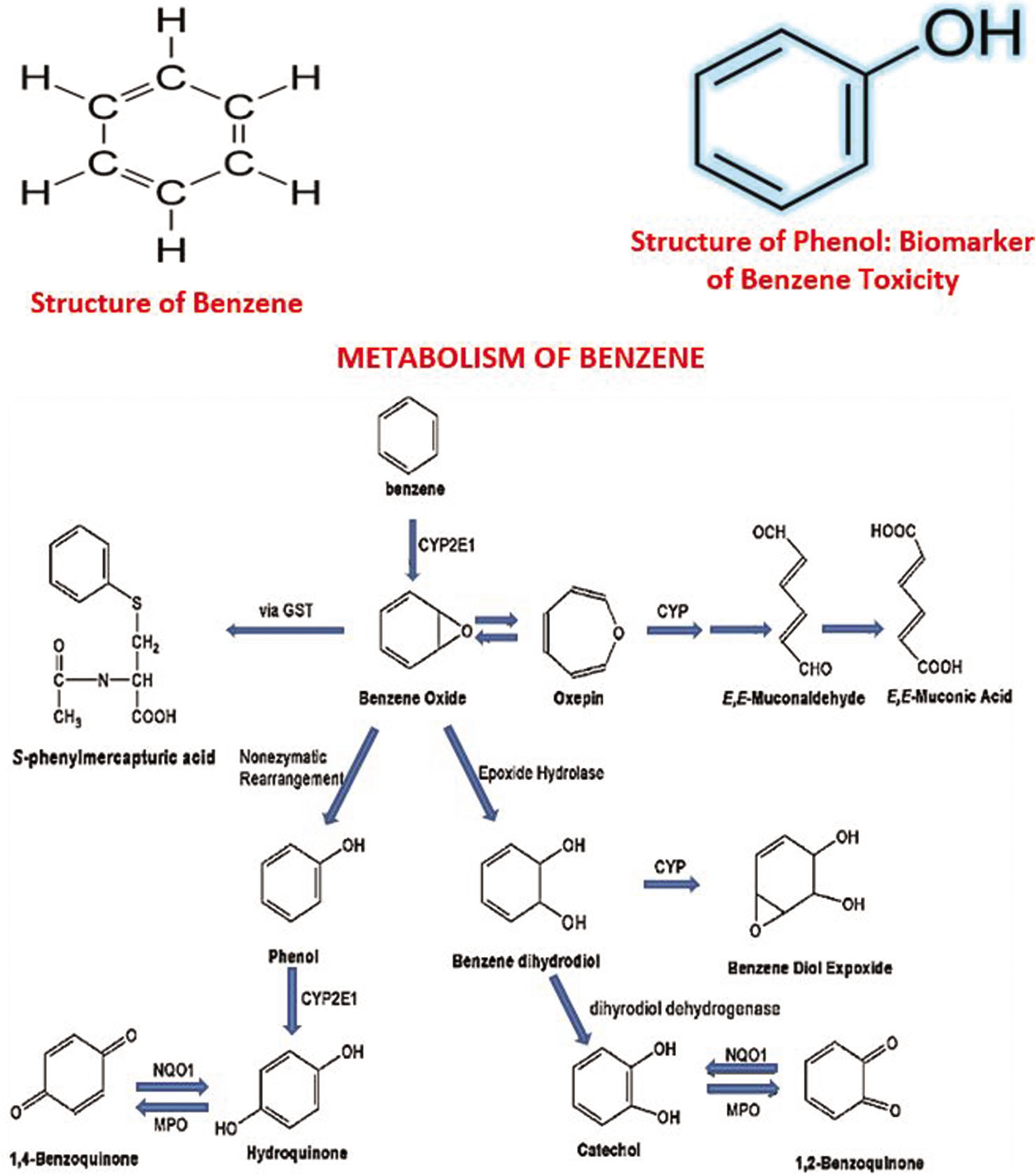
- The pathway of metabolism of benzene indicates that phenol is a key metabolite of benzene and serves as a key precursor of other bioactivated damaging reactive intermediates (metabolites) such as hydroquinone and 1,4-benzoquinone. The reader is referred to references 14 and 30 for an overview of benzene metabolism and data on its exposure and human risk assessment.
Examining our data in-depth; the significantly reduced serum Fe level in the GDs than that in controls may at least in part contribute to the disordered metabolism in the haemopoietic system that may through multiple steps culminate in myeloproliferative events. Fe is needed for the activity of enzymes such as catalase and peroxidase as well as for heme biosynthesis and the cytochromes.[8] Thus, this reduced Fe level may contribute to heme pathway diminution, instability, and susceptibility to derangement (heme pathway paresis).
This may be accentuated by the reduced Zn level which also plays a key role in heme biosynthesis; delta-aminolevulinic acid dehydratase (d-ALA-D) is Zn dependent.[37] The observed reduced heme level in the exposed GDs may be corroborative of the combined (synergistic adverse) effects of reduced Fe and Zn levels in the exposed workers. This appears to support earlier reports[38] in which there were decreased Fe concentrations in patients with acute myelogenous leukemia as well as acute lymphoblastic leukemia. The inverse correlation between urinary phenol and heme albeit not significant, may suggest a trend in the deleterious effects of metabolites of benzene on the haemopoietic system.
The significant reduction in serum Cu level and its inverse correlation with phenol appear to parallel that of Fe with which it is closely metabolically related, probably confirming the observations of previous investigators Maret,[36] about the mechanistic coordination of micronutrients. Evidence from animal models as well as from some human studies have shown that tumors contain high concentrations of Cu.[38] In contrast, this study showed a significantly lower serum Cu level in our study population than that of the unexposed. This finding also contradicts the report of some other investigators.[38] This discrepancy may reflect the degree or strength of the toxic metabolites generated in this study and demand for the bioavailability of Cu for protective mechanisms to mitigate the unfolding toxicity as enunciated below. The observed low serum Cu level in our study may reflect increased demand for Cu considering its role as an important antioxidant component of Cu, Zn-SOD and equally its involvement with ceruloplasmin as ferroxidase in an attempt to obtain more Fe bound to transferrin for ineffective erythropoiesis in a toxic metabolite burdened marrow. The reduced Cu level may additionally be reflective of the global role Cu plays in ensuring (boosting) total antioxidant capacity.[17,18] As a cofactor for superoxide dismutase, Cu helps prevent oxidative DNA damage by the superoxide anion O−2. Cu plays an important role in hemopoiesis thus reduced Cu bioavailability may compromise this process, impairing host defense. Cu deficiency also alters the levels of other cellular constituents involved in antioxidant activities such as selenium, and glutathione.[17] Cu is an integral part of varied enzymes involved in metabolic processes, matrix structures, and reactive oxygen species control, including ceruloplasmin (Feroxidase 1), cytochrome c oxidase, lysyl oxidase, tyrosinase, and amine oxidases.[17]
The described reduced serum Zn level in addition to the earlier discussion regarding d-ALAD may at least in part also be due to its increased consumption as part of Cu, Zn-SOD in combating toxic radicals induced by benzene metabolites as previously adduced for the reduced serum Cu level.[30] This reduced Zn level along with other members of the antioxidant defense system implies decreased scavenging of ROS and increased lipid peroxidation which are potential initiators of carcinogenesis.[18,39] A low Zn level also implies decreased DNA damage repair, impaired p53 function, poor cell cycle regulation, all of which are Zn-dependent.[40] Orlov et al.[40] indeed have elegantly enunciated the global control of Zn in conformation of a great number of proteins; transcription factors, signal pathway of apoptosis and complexes all of which will be deranged in Zn deficiency and permissive of risk of myeloproliferative disorders. Zn is of great significance in the process of hemopoiesis and has been described by Orlov et al.[40] as “almighty” and if its homeostasis is perturbed may lead to leukemia. The roles of Zn (II) ions in biological phosphorylations and redox signaling are well recognized and are among the spectrum of actions of Zn in cellular proliferation, differentiation, and cell death.[36] This may imply that tyrosine kinase activity of the protein, BCR-ABL required for oncogenic activity may be affected. Tyrosine kinase can become mutated and may be permanently turned “on.” Inhibition of this enzyme has been explored in imatinib, a tyrosine kinase inhibitor. Zn is not just required for the function of proteins it participates in cellular metabolism.[36]
In addition, ROS are recognized to activate nuclear factor-kappa B which may in turn activate growth factors; antiapoptotic molecules (BCL-2) permissive of cell proliferation, inflammatory cytokines, and adhesion molecules.[36,37,40] Consequently, the significantly lowered serum Zn level in these GDs and the significant inverse correlation observed between Zn and phenol are salient, given the numerous molecular and cellular activities in which Zn is involved, including genome stability. Carcinogenesis is a multistep process in which an accumulation of genetic events within the target organ or system may lead to progressive dysplastic cellular architecture, deregulated cell growth, and ultimately cancer. Aside from the possible direct effect of Zn in accumulated genetic events, Zn may also indirectly be involved in carcinogenesis through its regulation of vitamin A metabolism. Retinoids, including Vitamin A have anti-carcinogenic activities which may reflect the ability to induce differentiation. The bioavailability and utilization of Vitamin A depends on adequate Zn status[40] again underscoring the pivotal role of Zn in fundamental cellular processes and the pathologic implications of the observed Zn level in this study.
The p53 protein and other proteins that are involved in regulating HSC, proliferation through apoptosis, DNA repair and prevention of DNA damage are Zn -dependent.[37,40] Attenuation of apoptosis and accelerated tumor growth correlates with loss of p53 which can follow reduced Zn level. There could also be attendant oxidative damage to p53 due to the toxic metabolites induced oxidative stress. The p53 protein is one of the most vulnerable to oxidative stress.[41] The p53 protein is well known as the guardian of the genome.[40] The resulting loss of p53 activity secondary to Zn deficiency may lead to impairment of recognition of DNA damage, repair mechanisms and genome instability, precursor of carcinogenesis.[37] These have been directly or tangentially corroborated by previous reports[40] and elegantly supported by the recent in-depth review of Orlov et al.[40] in the pathway to leukemia. The report of Bhardwaj and Dhawan[38] who advocated the use of Zn in the treatment of another environmental toxicant induced hematological disorder; arsenic appears to lend support to our hypothesis.
Alterations in key micronutrient levels have been described in patients with leukemia[42] Indeed, Zuo et al.[42] suggested that changes in these essential trace element levels including selenium might be used as markers in clinical medicine for the diagnosis and prognosis of leukemia. Taken together, the data from this study have provided evidence that the perturbation of key micronutrients in the hemopoietic system, particularly Zn, precipitated by the toxic metabolites of benzene of which phenol is central leads to intense oxidative stress, in turn, leading to deranged DNA repair, impaired biological phosphorylation, altered signaling, and cell cycle deregulation leading to genome instability. This puts these GDs at increased risk of myelotoxicity and myeloproliferative disorders. A practical implication of this study is that evaluating the levels of these key heme pathway micronutrients along with phenol and heme levels may serve as risk assessment for this hematological malignancy. It may also lead to the development of chemopreventive agents in line with the suggestions of Zuo et al.,[42] Ibrahim,[28] and our earlier propositions on this,[18] thus of considerable public health significance, not only for occupationally exposed individuals but also the general population in emerging industrializing nations.
Weakness of the study and suggested further investigations
While we believe that the data from this study largely reflect the biological situation in the GDs, we must also acknowledge some weakness of the study. We recognized that urinary phenol has lower sensitivity, and that urinary benzene is a better biomarker. Furthermore, we did not determine hemoglobin level in our study participants, the report of Udonwa et al.[29] from the same environment showed dose dependent (duration of exposure dependent) decline in hematocrit, corroborating our heme levels and consequences. Regarding the levels of the micronutrients, though it is possible that socioeconomic status may have a role to play we consider that a remote possibility. Our previous studies revealed that Cu is actually higher in low socio economic (LSE) class than in the high socio-economic class (HSE) probably because Cu intake is readily available in nuts and pulses that are readily consumed by LSE individuals unlike HSE that consume more of dairy and protein rich diet. Similarly, we had previously reported that Zn varied more dose dependently than with SES in our previous cohort of workers occupationally exposed to lead.[18] Thus, it is most probable that the explanations offered in this study are among the most plausible. Though the referent subjects were educationally better off than these GDs it may not automatically translate to higher SES and better nutritional status. These may be considered preliminary findings awaiting further scientific confirmation that may include examination of better markers of clonal myeloproliferative disorders and genetic abnormality such as Philadelphia (ph) chromosome in targeted studies.
CONCLUSION
Benzene toxicity occurs in populations occupationally and environmentally exposed to gasoline which may predispose these individuals to risk of developing leukemia through mechanisms that may involve disruption of the central roles of key micronutrients in molecular events involved in hemopoiesis and the antioxidant system. The disruption of normal metabolism of the key micronutrients, Cu, Fe, and Zn, involved in the heme biosynthetic pathway and attendant oxidative stress, disruption of DNA repair, altered biological phosphorylation and impairment of cell cycle regulation by apoptosis as well as signaling occupies a central position here. Although these results may be considered preliminary data, they hold great promise for better understanding of benzene toxicity and chemopreventive potential. They may also lead to the development of chemopreventive agents in line with a position advanced over a decade ago and reaffirmed as well as the suggestions of other investigators and thus of considerable public health significance. They may not only apply to occupationally exposed individuals but also to the general population, particularly in the developing countries where the environmental prevalence of benzene from poor regulation is much higher than in the developed countries.
Ethical approval
The experimental design and protocol of this study was approved by the joint University of Ibadan and the University College Hospital (U.I./UCH) Ethics Committee; UI/EC/0165.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Occurrence and distribution of monocyclic aromatic hydrocarbons (BTEX) and the impact on macrobenthic community structure in Lagos Lagoon, Nigeria. Environ Monit Assess. 2016;188:571-88.
- [CrossRef] [PubMed] [Google Scholar]
- Elevated indoor volatile organic compound in the Niger Delta region of Nigeria of Nigeria. Int J Environ Res Public Health. 2018;15:1939-50.
- [CrossRef] [PubMed] [Google Scholar]
- Occupational exposure benzene, toluene and xylene (BTEX) to pump attendants in Ghana: Implications for policy guidance. Cog Environ Sci. 2019;5:1603418.
- [CrossRef] [Google Scholar]
- Improving and expanding estimates of the global burden of disease due to environmental health risks. Environ Health Perspect. 2019;127:105001.
- [CrossRef] [PubMed] [Google Scholar]
- Toxicological Profile of Benzene Atlanta, GA: U. S. Department of Health and Human Services; 2007.
- [Google Scholar]
- Global Chemical Outlook: Towards Sound Management of Chemicals Nairobi, Kenya: United Nations Environment Programme; 2012.
- [Google Scholar]
- Hematotoxicity: Chemically induced toxicity of the blood In: Williams PL, James RC, Roberts SM, eds. Principles of Toxicology: Environmental and Industrial Applications (2nd ed). New York: John Willey and Sons; 2000. p. :87-109.
- [CrossRef] [Google Scholar]
- Human benzene metabolism following occupational and environmental exposures. Chem Biol Interact. 2010;184:189-95.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic exposure to benzene: The clinical effects. J Ind Hyg Toxicol. 1939;21:331-54.
- [Google Scholar]
- A brief review of the relationship between occupational benzene exposure and hematopoietic cancer. Ann Occup Environ Med. 2018;30:33-7.
- [CrossRef] [PubMed] [Google Scholar]
- Generator diesel exhaust: A major hazard to health and environment in Nigeria. Am J Respir Crit Care Med. 2011;183:1437.
- [CrossRef] [PubMed] [Google Scholar]
- The use of biomonitoring data in exposure and human health risk assessment: benzene case study. Crit Rev Toxicol. 2013;43(2):119-153.
- [CrossRef] [PubMed] [Google Scholar]
- Retrospective estimation of exposure to benzene in a leukemia case-control study of petroleum marketing and distribution workers in the United Kingdom. Occup Environ Med. 1997;54:167-75.
- [CrossRef] [PubMed] [Google Scholar]
- Current understanding of the mechanism of benzene-induced leukemia in humans: Implications for risk assessment. Carcinogenesis. 2012;33:240-52.
- [CrossRef] [PubMed] [Google Scholar]
- Altered serum levels of elements in acute leukemia cases in Turkey. Asian Pac J Cancer Prev. 2011;12:3471-4.
- [Google Scholar]
- High cadmium/zinc ratio in cigarette smokers: Potential implications as a biomarker of risk of prostate cancer. Niger J Physiol Sci. 2008;23:41-9.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of benzene on human hematopoiesis. Open Hematol J. 2008;2:87-102.
- [CrossRef] [Google Scholar]
- Benzene and human health: A historical review and appraisal of associations with various diseases. Crit Rev Toxicol. 2010;40(Suppl 2):1-46.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanisms of induction of cytosolic and microsomal glutathione transferase (GST) genes by xenobiotics and pro-inflammatory agents. Drug Metab Rev. 2011;43:92-137.
- [CrossRef] [PubMed] [Google Scholar]
- Initial sequencing, and analysis of the human genome. Nature. 2001;409:860-921.
- [CrossRef] [PubMed] [Google Scholar]
- A colorimetric method for the determination of traces of phenol in water. Analyst. 1937;62:117-20.
- [CrossRef] [Google Scholar]
- Determination of phenols by the P-nitro-sodimethylaniline method. Sewage Ind Waste. 1952;24:1389-96.
- [Google Scholar]
- Clinical Biochemistry of Animals (4th ed). New York: Academic Press Incorporated; 1999. p. :932.
- [Google Scholar]
- Crystal spectra of a heme and some heme-protein complexes. Biochemistry. 1967;6:1563-6.
- [CrossRef] [Google Scholar]
- Practical Toxicology: Evaluation, Prediction and Risk. Boca Raton: CRC Press. Taylor and Francis Group 2017:344.
- [CrossRef] [Google Scholar]
- Protective effects of zinc and selenium against benzene toxicity in rats. Toxicol Ind Health. 2011;27:537-45.
- [CrossRef] [PubMed] [Google Scholar]
- Exposure of petrol station attendants and auto-mechanics to premium motor spirit fumes in Calabar, Nigeria. J Environ Public Health. 2009;2009:281876.
- [CrossRef] [PubMed] [Google Scholar]
- An overview of benzene metabolism. Environ Health Perspect. 1996;104(Supp6):1165-1171.
- [CrossRef] [PubMed] [Google Scholar]
- Using urinary biomarkers to elucidate dose-related patterns of human benzene metabolism. Carcinogenesis. 2006;27:772-81.
- [CrossRef] [PubMed] [Google Scholar]
- Personal exposure to different levels of benzene and its relationships to the urinary metabolites S-phenylmercapturic acid and transmuconic acid. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;778:211-21.
- [CrossRef] [Google Scholar]
- Mechanism of action of benzene toxicity: Cell cycle suppression in hemopoietic progenitor cells (CFU-GM) Exp Hematol. 2001;29:278-85.
- [CrossRef] [Google Scholar]
- Chemically induced carcinogenesis in rodent models of aging: Assessing organismal resilience to genotoxic stressors in geroscience research. Geroscience. 2019;41:209-27.
- [CrossRef] [PubMed] [Google Scholar]
- Tissue-specific metabolism of benzene in Zymbal gland and other solid tumor target tissues in rats. J Am Coll Toxicol. 1995;14:40-60.
- [CrossRef] [Google Scholar]
- Zinc biochemistry: From a single enzyme to a key element of life. Adv Nutr. 2013;4:82-91.
- [CrossRef] [PubMed] [Google Scholar]
- Zinc deficiency induces oxidative DNA damage and increased p53 expression in human lung fibroblast. J Nutr. 2003;133:2543-8.
- [CrossRef] [PubMed] [Google Scholar]
- Zinc treatment modulates hematological and morphological changes in rat erythrocytes following arsenic exposure. Toxicol Ind Health. 2019;35:593-603.
- [CrossRef] [PubMed] [Google Scholar]
- Topoisomerase II inhibition by myeloperoxidase-activated hydroquinone: A potential mechanism underlying the genotoxic and carcinogenic effects of benzene. Chem Biol Interact. 2010;153:207-16.
- [CrossRef] [PubMed] [Google Scholar]
- The role of zinc and its compounds in leukemia. J Biol Inorg Chem. 2018;23:347-62.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of inorganic nutrients on maintenance of genome instability. Environ Mol Mutagen. 2009;50:349-60.
- [CrossRef] [PubMed] [Google Scholar]
- Levels of selenium, zinc, copper and antioxidant enzyme activities in patients with leukemia. Biol Trace Elem Res. 2006;114:41-53.
- [CrossRef] [Google Scholar]






