Translate this page into:
Transdermal antimalarial drug delivery to improve poor adherence to antimalarials: A new light at the end of the tunnel

*Corresponding author: Uzochukwu Emmanuel Chima, Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka, Enugu State, Nigeria. uzochukwu.chima.232897@unn.edu.ng
-
Received: ,
Accepted: ,
How to cite this article: Agbo CE, Chima UE, Ogbobe SC, Omotayo FO, David SC. Transdermal antimalarial drug delivery to improve poor adherence to antimalarials: A new light at the end of the tunnel. Am J Biopharm Pharm Sci 2023;3:4. doi: 10.25259/AJBPS_14_2023
Abstract
Malaria, a perilous disease caused by Plasmodium parasites and characterized by a substantial mortality rate, has persistently posed as a global health challenge. Conventional antimalarial formulations, although effective, grapple with issues surrounding their bioavailability and palatability, and potentially hampering patient adherence and inadvertently fueling drug resistance and poor treatment outcomes. This paper meticulously delves into the predicaments associated with prevailing antimalarial delivery methods – oral, intravenous, and intramuscular. The paper navigates through the compelling merits of the transdermal pathway, drawing inspiration from its triumphant deployment in other medical realms. The investigation extends to encompass preclinical inquiries dedicated to exploring the transdermal administration of antimalarials. Transdermal antimalarials have shown complete suppression and elimination of Plasmodium parasites, as suggested by the preclinical studies. These preclinical studies emerge as a beacon of hope, exhibiting heightened bioavailability, enhanced safety margins, and notable cost-effectiveness when compared with oral antimalarials. Moreover, this innovative avenue for drug delivery not only offers convenience but also holds the potential to be a transformative solution to the adherence problems of traditional antimalarials, which currently afflicts standard therapeutic options.
Keywords
Transdermal
Antimalarial
Drug resistance
Adherence
Drug delivery
INTRODUCTION
Malaria is the deadliest parasitic disease, with 247 million cases and 619,000 deaths worldwide in 2021, including 274,030 children under the age of 5, yet available treatments are prone to drug resistance and lack palatability. The majority of malaria cases were recorded on the African continent.[1] Current antimalarials medications are administered either orally, intramuscularly, or intravenously. However, the drug efficacy and patient compliance of these conventional antimalarials therapies are hindered by emerging resistance by the parasite as well as lack of palatability, especially for the oral medications, negatively impacting efforts toward prevention, control, and utmost eradication of malaria. A study showed that more than 90% of the respondents following an antimalarial therapy felt that the medications had an unpleasant taste and that taking them was an uncomfortable experience. As a result, patients are more likely to stop taking their medication,[2] leading to poor adherence and development of drug resistance.[3]
Herein, our study intends to harness the potential benefits of the transdermal administered antimalarial solutions in addressing issues with palatability, adherence, and antimalarial drug resistance. We aim to explore the successful preclinical trials done so far on transdermal antimalarial therapies and suggest the possible commencement of clinical trials in humans.
Problems of Current Routes of Antimalarials
Despite the advancements in antimalarials therapies, malaria morbidity and mortality are still on the increase. This has mostly been attributed to the emergence and transmission resistance of the Plasmodium parasite to the drugs, which results from non-adherence to the traditional oral and parenteral antimalarials. Furthermore, non-adherence is associated with the bad or unpleasant taste of most oral antimalarials. In a study carried out by Afaya et al., over 90% of respondents agreed that antimalarials medications had bad taste, and thus, they had an unpleasant feeling while taking them.[2] One of the main barriers to uptake and adherence to prophylactic antimalarial medications among the African community in London is poor taste.[4] Lawford et al. observed that patients who dislike the taste or smell of Artemether (ART)-lumefantrine (LUM) were less likely to be completely adherent.[5] According to Matsui, non-adherence can compromise the efficacy of medication regimens, leading to failure to achieve the desired treatment goal.[6] Thus, non-adherence to antimalarials may lead to significant morbidity, wasted resources and/or resistance to these medications. In addition, poor adherence to oral antimalarials can be caused by medication overload or frequent dosing.[2,7] Individuals often have a preference for specific antimalarial drugs, particularly those that come with simple dosing instructions.[8,9] Furthermore, the therapeutic efficacy of artemisinins is constrained due to their inadequate bioavailability and restricted pharmacokinetics.[10] Oral antimalarials are faced with issues of poor bioavailability. Newton et al. found that the bioavailability of artesunate is only 61%.[11] Poor adherence to injectable antimalarials could be due to reaction at the site of injection and pain. In a research work carried out by Ampadu et al., there was low adherence to injectable antimalarials.[12] Since most antimalarials are administered through this route, there is a need to devise a better route that would promote adherence and curb resistance. This poor adherence to these traditional antimalarials plays a role in the future drug resistance by the Plasmodium parasites. As such, identifying ways to improve adherence and bioavailability with these antimalarials will help mitigate drug resistance.[2] Therefore, an alternative route of administration that could curb these challenges should be employed to drastically mitigate these problems.
TRANSDERMAL ROUTE AS A SOLUTION
Transdermal drug delivery system (TDDS, patch) is a promising, alternative, and innovative route to the conventional drug delivery routes of oral ingestion and parenteral administration. Its unique advantages include noninvasiveness, prolonged duration of action, avoidance of the first pass effect, reduced dosing frequency, and improved patient compliance, the flexibility of terminating drug administration by removing the patch among others.[13] Regardless of its few disadvantages which include the skin’s low permeability due to the barrier offered by the stratum corneum and local irritation at the site of application,[13] notable success has been recorded with the TDDS since the commercial use of Transderm-Scop® patch in the United States of America (US) – the first widely recognized TDDS indicated for motion sickness.[14]
Several other drugs have been formulated into a patch to mitigate against the limitations of their traditional forms [Table 1]; the Nitro-Dur I® (developed by Key Pharmaceuticals), Transderm-Nitro® (by Ciba Pharmaceuticals Company), and Nitrodisc® (Searle Laboratories) were all introduced in 1981 for the prevention and treatment of angina pectoris with an advantage of reduced dosing frequency;[14] transdermal clonidine for the treatment of hypertension with an advantage of reduced side effects and improved patient compliance;[14] and nicotine patches such as Habitrol®, Nicotrol®, Nicoderm®, and Prostep® developed for smoking cessation. This was popularly regarded as the first transdermal blockbuster due to its outstanding milestone where over 1 million smokers quit smoking.[14] In all these transdermal products, the advantages necessitate their delivery as patches. The different techniques for the enhancement of transdermal drug delivery also hold promise for these advantages.
These techniques gave rise to three generations of TDDS [Figure 1].[15]
| Brand name | Drug | Manufacturer | Indications |
|---|---|---|---|
| Nicotinell® | Nicotine | Novartis | Pharmacological smoking cessation |
| Matrifen® | Fentanyl | Nycomed AB | Pain relief patch |
| Ortho Evra® | Norelgestromin/ethinyl estradiol | ORTHO-McNEIL | Post-menopausal syndrome |
| NuPatch 100 | Diclofenac diethylamine | Zydus Cadila | Anti-Inflammatory |
| Neupro® | Rotigotine | UCB and Schwarz Pharma | Early-stage idiopathic Parkinson’s disease |
| Alora | Estradiol | TheraTech/Procter and Gamble | Post-menopausal syndrome |
| Nicoderm® | Nicotine | Alza/GlaxoSmithKline | Smoking cessation |
| Estraderm | Estradiol | Alza/Novartis | Post-menopausal syndrome |
| Climara | Estradiol | 3M Pharmaceuticals/BerlexLabs | Post-menopausal syndrome |
| Androderm | Testosterone | TheraTech/GlaxoSmithKline | Hypogonadism in males |
| Nitrodisc | Nitroglycerin | Roberts Pharmaceuticals | Angina pectoris |
| Transderm-Scop® | Scopolamine | Alza/Novartis | Motion sickness |
| Nuvelle TS | Estrogen/Progesterone | Ethical Holdings/Schering | Hormone replacement therapy |
| Deponit | Nitroglycerin | Schwarz-Pharma | Angina pectoris |
| Nitro-dur | Nitroglycerin | Key Pharmaceuticals | Angina pectoris |
| Catapres TTS® | Clonidine | Alza/Boehringer Ingelheim | Hypertension |
| FemPatch | Estradiol | Parke-Davis | Post-menopausal syndrome |
| Minitran | Nitroglycerin | 3M Pharmaceuticals | Angina pectoris |
| Duragesic® | Fentanyl | Alza/Janssen Pharmaceutical | Moderate/severe pain |
| Estraderm | Estradiol | Alza/Novartis | Post-menopausal syndrome |
| Fematrix | Estrogen | Ethical Holdings/Solvay Healthcare Ltd. | Post-menopausal syndrome |
| Transderm-Nitro® | Nitroglycerin | Alza/Novartis | Angina pectoris |
| Oxytrol® | Oxybutynin | Watson Pharma | Overactive bladder |
| Prostep | Nicotine | Elan Corp./Lederle Labs | Smoking cessation |
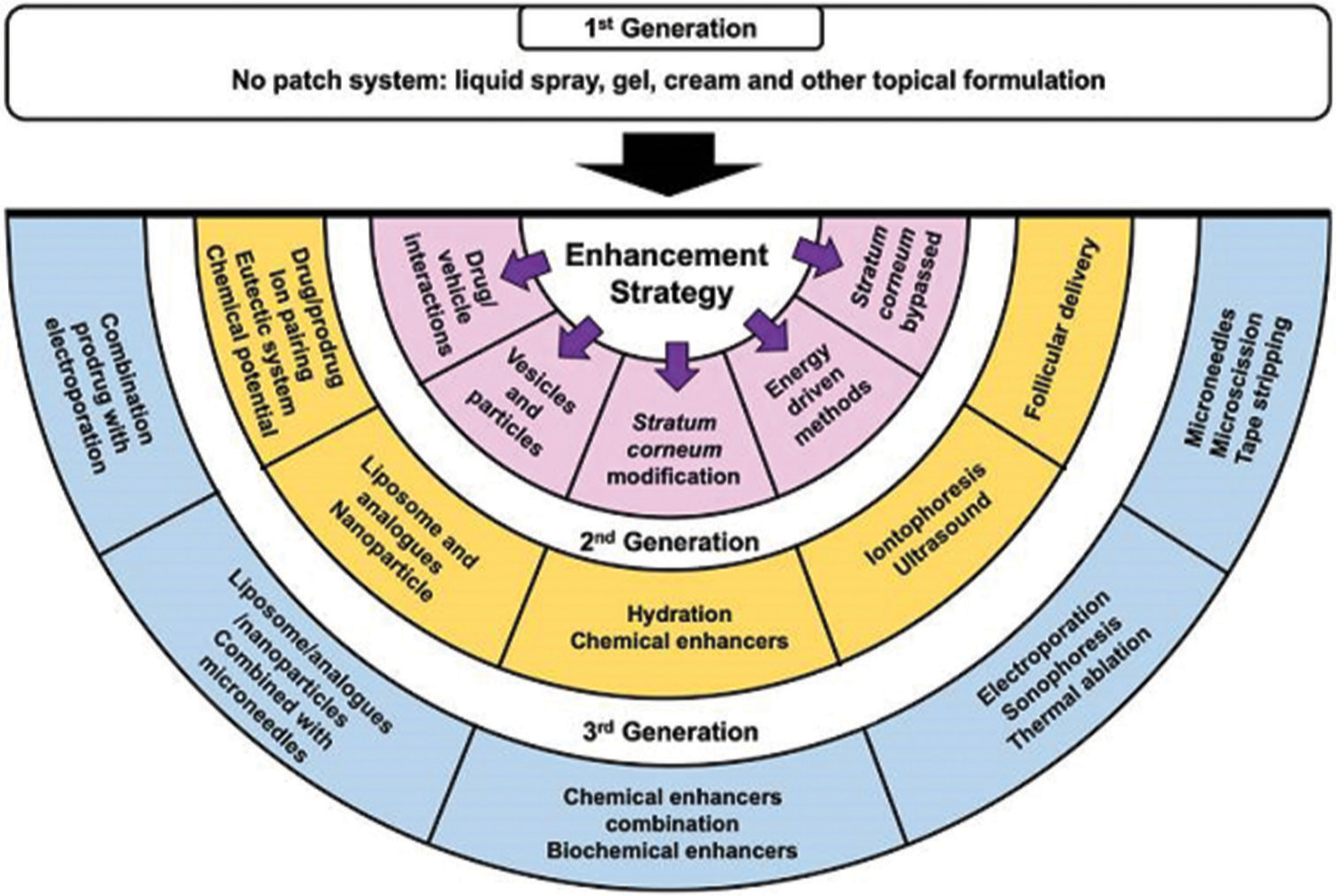
- Techniques utilized for enhancing transdermal drug delivery.
First-generation delivery systems
They rely mostly on suitable drug characteristics that allow skin absorption without appreciably enhancing skin penetration and do not involve any patch system, drug is formulated into a typical liquid spray, gel, cream, or other topical formulations. Thus, this generation employs passive diffusion in achieving absorption of the drug and characteristic drugs must be of low molecular mass (<600 Da), high lipophilicity, and effective in low dose.[16]
Second-generation delivery systems
They aimed at enhancing skin permeability by adjusting the stratum corneum and applying a driving force (energy-driven methods) to the skin’s surface while avoiding harm to the skin’s deeper layers. This generation typically involves both skin hydration methods using various chemical enhancers and electrically assisted methods through iontophoresis in enhancing the absorption of the drugs into the skin.[17,18]
Third-generation delivery systems
They also employ energy-driven methods aimed at disrupting or removing the stratum corneum without causing damage to the deeper layers of the skin. Such techniques include microchannel creation as in electroporation and stratum corneum removal as in microscissioning.[15,16,18] Combinations of chemical enhancers, biochemical enhancers, cavitational ultrasound, and microneedles (vaccine delivery) also characterize this generation[15] and they particularly enable transdermal delivery of macromolecules and vaccines.
Antimalarials are not left out in this possibility especially as numerous limitations are building up against the most widely used antimalarial agents – the artemisinin-combination therapies (ACTs); resistance, low bioavailability, and patient non-adherence being the most notable. On this note, several attempts have been made to develop new antimalarial drugs and novel delivery systems, of which transdermal patches are the most mooted option.
Many preclinical studies have revealed promising benefits with transdermal antimalarials. A skin spray of artemisone – a relatively new artemisinin derivative[19] formulated as microemulsions (ART-ME) revealed a high transcutaneous bioavailability and, most significantly, showed complete elimination of Plasmodium Berghei, and prevented cerebral malaria (dose: 13.3 mg/kg) when compared to artesunateME (dose: 40 mg/kg), untreated, and placebo-treated mice.[20] A similar study also revealed significant suppression of P. berghei for transdermal asiatic acid-pectin patch (5 mg/ kg) than chloroquine.[21] Increased bioavailability was noticed in a study that identified transdermal surfactant-based ART-LUM (S3AL) as a potential new candidate for the treatment of malaria,[22] thus solving the problems of the low solubility of oral-based therapy ART and LUM. In addition to the aforementioned advantages of TDDS, a cost-effective transdermal bio-adhesive containing ART showed higher absorption and permeation rates,[23] highlighting transdermal antimalarials as a cheap and safe option for patients from low-resource areas. It is imperative to note that following the potentials offered by the various strategies for the enhancement of TDDS as discussed earlier, the properties of the third-generation delivery systems – enabling the transdermal delivery of macromolecules – could be applied to these potential transdermal antimalarial drugs as they are stepped up to human clinical trials.
TOXICOLOGICAL STUDIES OF THE TRANSDERMAL ROUTE
The transdermal route is considered generally safe but not devoid of possible toxicities. Volpe-Zanutto et al. showed that the S3AL formulation, out of other formulations used in the study, was found to be the most promising option for a transdermal system based on surfactants in the toxicological investigations.[23] In this formulation, LUM and ART are combined in a liquid crystal system. At concentrations of 1.95 µg/mL of the formulation, almost 80% of human keratinocyte cells were still alive, demonstrating its low toxicity and promise as a drug candidate.[22] In Ananda et al.’s preclinical study involving a combination of transdermal patch and solid microneedles for the transdermal delivery of Primaquine, histopathological analyses confirmed the results of in vitro hemolytic and in vivo irritation tests, which were used to assess the safety of the transdermal patch. Results show hemolysis of less than 5% and no significant damage, which highlights the safety of this approach.[24] In addition, a skin tolerance test was conducted for the nanogel transdermal formulation of ART, and following a 5-day treatment period, no signs of skin reaction, including redness/erythema, wrinkles, papules, and/or dermatitis, were noted.[25] The transdermal antimalarial route has shown to be safe and less toxic. However, it could have common complications faced by the general transdermal route, namely, skin irritation and hypersensitivity and drug accumulation on the skin and patch residue; thus, human clinical trials could offer further perspective on the toxicity profile of these potential transdermal antimalarials.
CONCLUSION
Transdermal delivery offers a viable alternative to oral administration of drugs. Unlike oral delivery, it does not pose adherence issues and can be self-administered. This method enables the sustained release of medications over extended periods, leading to improved patient adherence. In addition, transdermal systems are cost-effective. Numerous drugs have been successfully formulated into patches, overcoming the limitations associated with their traditional forms. However, antimalarials face various challenges when administered orally, even the most widely used antimalarial agents. To address these limitations, formulating antimalarial drugs as transdermal patches, especially while integrating the characteristics of third-generation delivery systems, presents a potential solution as suggested in successful preclinical studies. By doing so, some of the side effects associated with certain antimalarial agents can be mitigated. Transdermal patches offer several advantages, including enhanced safety, improved bioavailability, affordability, and easy accessibility for individuals. This approach has the potential to provide a safe and convenient option for delivering antimalarial medications; thus, clinical trials could be the route to seeing the light at the end of the malaria tunnel.
Authors’ contributions
Dr. Chinonyelum Emmanuel Agbo and Dr. Uzochukwu Emmanuel Chima conceptualized and designed this work. All the authors were involved in drafting the full manuscript.
Ethical approval
Not applicable.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- World malaria report 2022. 2022 Available from: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021 [Last accessed on 2023 Jul 16]
- [Google Scholar]
- Patients' adherence to antimalarial medication; Self-report of patients at the Volta regional hospital of Ho, Ghana. Int J Res Med Sci. 2017;5:4234-41.
- [CrossRef] [Google Scholar]
- Antimalarial drug resistance in Africa: Key lessons for the future. Ann N Y Acad Sci. 2015;1342:62-7.
- [CrossRef] [PubMed] [Google Scholar]
- Barriers to uptake and adherence with malaria prophylaxis by the African community in London, England: Focus group study. Ethn Health. 2005;10:355-72.
- [CrossRef] [PubMed] [Google Scholar]
- Adherence to prescribed artemisinin-based combination therapy in Garissa and Bunyala districts, Kenya. Malar J. 2011;10:281.
- [CrossRef] [PubMed] [Google Scholar]
- Drug compliance in pediatrics. Clinical and research issues. Pediatr Clin North Am. 1997;44:1-14.
- [CrossRef] [PubMed] [Google Scholar]
- Perceptions and utilization of the anti-malarials artemether-lumefantrine and dihydroartemisininpiperaquine in young children in the Chikhwawa district of Malawi: A mixed methods study. Malar J. 2015;14:13.
- [CrossRef] [PubMed] [Google Scholar]
- Local perceptions of intermittent screening and treatment for malaria in school children on the South coast of Kenya. Malar J. 2012;11:185.
- [CrossRef] [PubMed] [Google Scholar]
- Adherence to treatment with artemether-lumefantrine for uncomplicated malaria in rural Malawi. Clin Infect Dis. 2011;53:772-9.
- [CrossRef] [PubMed] [Google Scholar]
- Identification of an antimalarial synthetic trioxolane drug development candidate. Nature. 2004;430:900-4.
- [CrossRef] [PubMed] [Google Scholar]
- Antimalarial bioavailability and disposition of artesunate in acute falciparum malaria. Antimicrob Agents Chemother. 2000;44:972-7.
- [CrossRef] [PubMed] [Google Scholar]
- Prescribing patterns and compliance with World Health Organization recommendations for the management of severe malaria: A modified cohort event monitoring study in public health facilities in Ghana and Uganda. Malar J. 2019;18:36.
- [CrossRef] [PubMed] [Google Scholar]
- PharmaTutor. Available from: https://www.pharmatutor.org/articles/transdermal-drug-delivery-system-a-total-view [Last accessed on 2023 Jun 21]
- [Google Scholar]
- Transdermal patches: History, development and pharmacology. Br J Pharmacol. 2015;172:2179-209.
- [CrossRef] [PubMed] [Google Scholar]
- Enhancement strategies for transdermal drug delivery systems: Current trends and applications. Drug Deliv Transl Res. 2022;12:758-91.
- [CrossRef] [PubMed] [Google Scholar]
- Permeation enhancer strategies in transdermal drug delivery. Drug Deliv. 2016;23:564-78.
- [CrossRef] [PubMed] [Google Scholar]
- Innovative strategies for enhancing topical and transdermal drug delivery. Open Drug Deliv J. 2007;1:36-59.
- [CrossRef] [Google Scholar]
- First assessment in humans of the safety, tolerability, pharmacokinetics, and ex vivo pharmacodynamic antimalarial activity of the new artemisinin derivative artemisone. Antimicrob Agents Chemother. 2008;52:3085-91.
- [CrossRef] [PubMed] [Google Scholar]
- Transdermal delivery of artemisinins for treatment of pre-clinical cerebral malaria. Int J Parasitol Drugs Drug Resist. 2021;16:148-54.
- [CrossRef] [PubMed] [Google Scholar]
- Asiatic acid-pectin hydrogel matrix patch transdermal delivery system influences parasitaemia suppression and inflammation reduction in P. berghei murine malaria infected Sprague-Dawley rats. Asian Pac J Trop Med. 2016;9:1172-80.
- [CrossRef] [PubMed] [Google Scholar]
- Novel transdermal bioadhesive surfactant-based system for release and solubility improvement of antimalarial drugs artemether-lumefantrine. Biomed Mater. 2021;16(6) doi: 10.1088/1748-605X/ac2885
- [CrossRef] [PubMed] [Google Scholar]
- Semisynthetic derivative of Artemisia annua-loaded transdermal bioadhesive for the treatment of uncomplicated malaria caused by Plasmodium falciparum in children. J Pharm Sci. 2019;108:1177-88.
- [CrossRef] [PubMed] [Google Scholar]
- Combination of transdermal patches and solid microneedles for improved transdermal delivery of primaquine. Int J Pharm. 2021;609:121204.
- [CrossRef] [PubMed] [Google Scholar]
- Formulation and evaluation of transdermal nanogel for delivery of artemether. Drug Deliv Transl Res. 2021;11:1655-74.
- [CrossRef] [PubMed] [Google Scholar]







