Translate this page into:
Novel approaches to treat primary hyperlipidemia

*Corresponding author: Jaydeep Maganbhai Vachhani, Department of Pharmacology, School of Pharmacy, RK University, Rajkot, Gujarat, India. jp385719@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Vachhani JM, Tirgar PR. Novel approaches to treat primary hyperlipidemia. Am J Biopharm Pharm Sci. 2024;4:5. doi: 10.25259/AJBPS_7_2024
Abstract
Primary hyperlipidemias encompass a diverse range of genetic and multifactorial disorders characterized by heightened levels of cholesterol and/or triglycerides, typically manifesting early in life and often linked with familial predisposition. Despite their significant cardiovascular and pancreatic implications, a minority of cases are correctly identified and managed. This review aims to provide an updated overview of emerging therapeutic interventions for primary hyperlipidemia. Recent approvals from regulatory bodies such as the U.S. Food and Drug Administration and the European Medicines Agency have introduced novel lipid-lowering agents targeting key metabolic pathways. These include bempedoic acid, which inhibits adenosine 5'-triphosphates-citrate lyase, inclisiran, targeting proprotein convertase and subtilisin/kexin 9, addressing apolipoprotein CIII, and angiopoietin-like 3. Complementary to existing treatments such as statins, ezetimibe, and fibrates, these medications offer promising adjunctive effects. The potential clinical applications of these innovative therapies envisaging improved treatment outcomes and expanded options, particularly for patients who are facing negative consequences with current regimens. Integrating the new agents into the therapeutic armamentarium holds the potential to enhance treatment efficacy and safety profiles, advancing the administration of primary hyperlipidemia.
Keywords
Familial hypercholesterolemia
Familial chylomicronemia
Familial combined hyperlipidemia
Hydroxymethylglutaryl-CoA reductase inhibitors
Lipoprotein lipase activators
INTRODUCTION
Primary hyperlipidemias encompass a diverse spectrum of genetic and multifactorial conditions characterized by familial clustering, presenting severe hypercholesterolemia and/or hypertriglyceridemia, often emerging early in life, and posing a heightened risk of cardiovascular events or recurrent pancreatitis.[1-3] Prolonged exposure to elevated atherogenic particles predisposes these patients to premature and severe atherosclerosis.[4,5] Evidence from studies in familial hypercholesterolemia (FH) cohorts strongly advocates for initiating low-density lipoprotein cholesterol (LDL-C) reduction interventions at the earliest possible stage.[6,7] Aggressive LDL-C lowering, particularly initiated in adolescence, can mitigate cardiovascular mortality to levels comparable to the general population; conversely, delaying treatment until early adulthood yields diminished benefits.[8] Conversely, primary hypertriglyceridemias are linked to a higher risk of acute and recurrent pancreatitis,[9,10] surpassing that of secondary causes of extreme hypertriglyceridemia.[11]
PRIMARY HYPERLIPIDEMIA: CONCEPT AND TREATMENT PECULIARITIES
Optimal management of primary dyslipidemia entails early diagnosis through cascade screening and coordinated multidisciplinary care.[12,13] Patients should be educated about the significance of long-term adherence to therapy and lifestyle modifications,[14] though adherence may be challenging, particularly during adolescence and young adulthood.[15] While dietary modifications, especially fat and simple carbohydrate restriction, are pivotal for severe hypertriglyceridemia,[16] they are insufficient to achieve LDL-C targets in severe hypercholesterolemia, necessitating pharmacological interventions.[17] Careful family planning is advised due to potential negative impacts of hormonal contraceptives on lipid control,[18] with associated implications for medical insurance costs.
In reality, only a small fraction of primary hyperlipidemia cases receive proper recognition and treatment within most healthcare systems.[19,20] Effective cardiovascular prevention mandates a lifelong LDL-C reduction of 50% from baseline, aiming for LDL-C levels <55 or <70 mg/dL for extremely high or high coronary heart disease risk, respectively.[21,22] However, stringent targets[23] are often elusive due to a number of variables, such as the severity of the condition, lack of awareness, treatment costs, adverse drug reactions, nocebo effects, and systemic health-care challenges.[24-27] Addressing these limitations necessitates training for health-care professionals and patients, emphasizing standardized protocols.[28]
This article aims to provide an updated overview of current and emerging therapies for primary hyperlipidemia, highlighting recent approvals by regulatory authorities such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency. These novel lipid-lowering medications, targeting metabolic pathways including adenosine 5'-triphosphate (ATP)-citrate lyase (bempedoic acid [BA]), proprotein convertase subtilisin/kexin 9 (PCSK9; inclisiran), and angiopoietin-like 3 (ANGPTL3; evinacumab), offer additional benefits when used in conjunction with existing therapies such as statins, ezetimibe, or fibrates, potentially enhancing overall cardiovascular risk reduction and providing more comprehensive lipid management options.[29] The potential clinical indications for these innovative medications are discussed, envisioning improved treatment outcomes and expanded treatment choices for individuals with primary hyperlipidemia.
An extensive assessment of the literature was done utilizing PubMed and ClinicalTrials.gov databases, employing MESH codes “primary hyperlipidemia” and “treatment,” alongside specific keywords including “inclisiran,” and “bempedoic acid.” The search spanned from 2016 to 2021 and was executed collaboratively by two authors (C.A.A-S. and R.A.G-D.), with validation achieved through consensus among all three authors. Out of 1249 initially identified publications, only 144 were found appropriate for in-depth review. Excluded were thematic reviews, case reports, consensus documents, or studies not primarily focusing on primary hyperlipidemia cases. The review prioritized preclinical and clinical findings, international randomized controlled trials, and observational studies conducted among those suffering with primary dyslipidemias, particularly FH and primary chylomicronemias. In addition, the scope extended to encompass familial lipodystrophies and familial dysbetalipoproteinemia.[30] Community-based reports were included but predominantly centered on FH.[31]
CURRENTLY AVAILABLE TREATMENT APPROACHES FOR PRIMARY HYPERLIPIDEMIA
The administration of primary hyperlipidemia necessitates a multifaceted approach involving a healthy lifestyle, dietary modifications, regular exercise, and the application of lipid-lowering medications to achieve treatment goals [Figure 1]. Unlike conditions such as FH, familial combined hyperlipidemia, and dysbetalipoproteinemia, primary chylomicronemias typically do not present a higher cardiovascular events rate.[32] Instead, the focus is on preventing recurrent pancreatitis, attaining genetically determined height, and maintaining a healthy nutritional status. Conversely, the primary goals for polygenic severe hypertriglyceridemias are cardiovascular prevention and the prevention of pancreatitis events.[33]
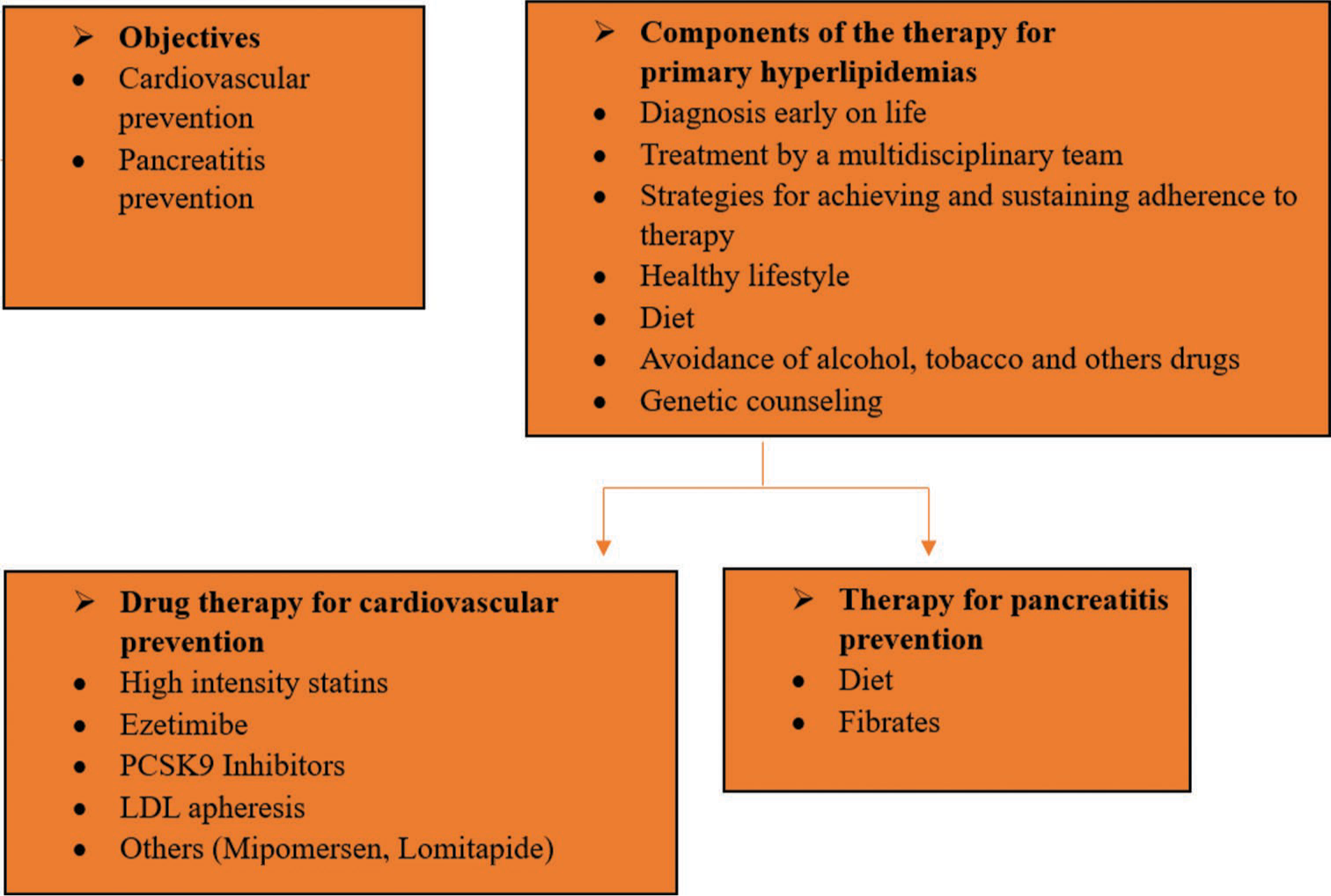
- Principles of the administration of primary hyperlipidemia. ANGPTL3: Angiopoietin-like protein 3, PCSK9: Proprotein convertase subtilisin-kexin type 9.
Statins remain the cornerstone intervention for preventing and treating atherosclerotic vascular disease,[34] with a 21% reduction in cardiovascular events observed per 1 mmol/L (38.6 mg/dL) reduction in LDL-C.[35] High-intensity statin treatment, like atorvastatin 40/80 mg or rosuvastatin 20/40 mg/day, is recommended initially, with consideration given to the highest tolerated dose due to statin intolerance and nocebo effects. A significant proportion of patients may require additional lipid-lowering agents, particularly in FH datasets (40–60%).[36] Ezetimibe is the preferred choice to combine with the highest tolerated statin dosage if LDL-C goals are not met,[20,22] offering an additional 21–27% LDL-C reduction,[37] with an excellent safety profile and relatively low cost.
PCSK9 inhibitors serve as a secondary option for combination therapy, significantly reducing LDL-C by as much as 60% regardless of concomitant lipid-lowering therapy. PCSK9 inhibitors have shown benefits in reducing cardiovascular events, even though more research is required to evaluate their influence on cardiovascular prevention in primary dyslipidemia. LDL-apheresis is effective in homozygous FH (HoFH) cases and some severe FH cases, but its widespread use is limited by high cost and limited access.[38]
Treatment for monogenic chylomicronemia primarily involves a very low-fat diet,[16] whereas polygenic severe hypertriglyceridemia management focuses on restricting simple carbohydrates, saturated, and polyunsaturated fats.[19] Nutritional guidance by a nutritionist is essential, with medium-chain triglycerides potentially offering benefits, especially in specific demographics.[39] At present, available lipid-lowering drugs play a secondary role in their treatment, with fibrates being the first option.[40] However, fibrates are not useful in monogenic chylomicronemias due to a compromised lipoprotein lipase (LPL) activity.[41] Plasmapheresis may be used during acute pancreatitis episodes, but conservative management typically yields better results at a lower cost.[42] LPL gene therapy (alipogene tiparvovec) was used in genetically proven LPL deficiency but was withdrawn from the market due to its high cost.[43]
NEW AND UPCOMING DRUG THERAPIES FOR PREVENTION OF CARDIOVASCULAR EVENTS
Recently, innovative treatment alternatives have emerged targeting complementary metabolic pathways to the statin-induced inhibition of hydroxy methyl glutaryl coenzyme A reductase [Figure 2]. Among these, BA inhibits (ATP)-citrate lyase, inclisiran targets PCSK9 expression. Notably, BA has received FDA permission to treat adult patients who are heterozygous FH (HeFH) or with a history of atherosclerotic heart disease not reaching the LDL-C treatment goal. Administered orally, BA exhibits a moderate effect on LDL-C and C-reactive protein (CRP), an inflammation biomarker.[44] Mechanistically, BA acts within the cholesterol synthesis pathway by inhibiting ATP-citrate lyase, an enzyme catalyzing an earlier step than hydroxymethylglutaryl coenzyme A reductase. This inhibition may enhance LDL receptors (LDLR) expression on hepatocyte membranes. BA, a prodrug converted to bempedoyl coenzyme, displays low rates of myopathic adverse events due to its selective conversion by acyl-coenzyme A synthetase-1, predominantly discovered in the liver and kidney.[45]
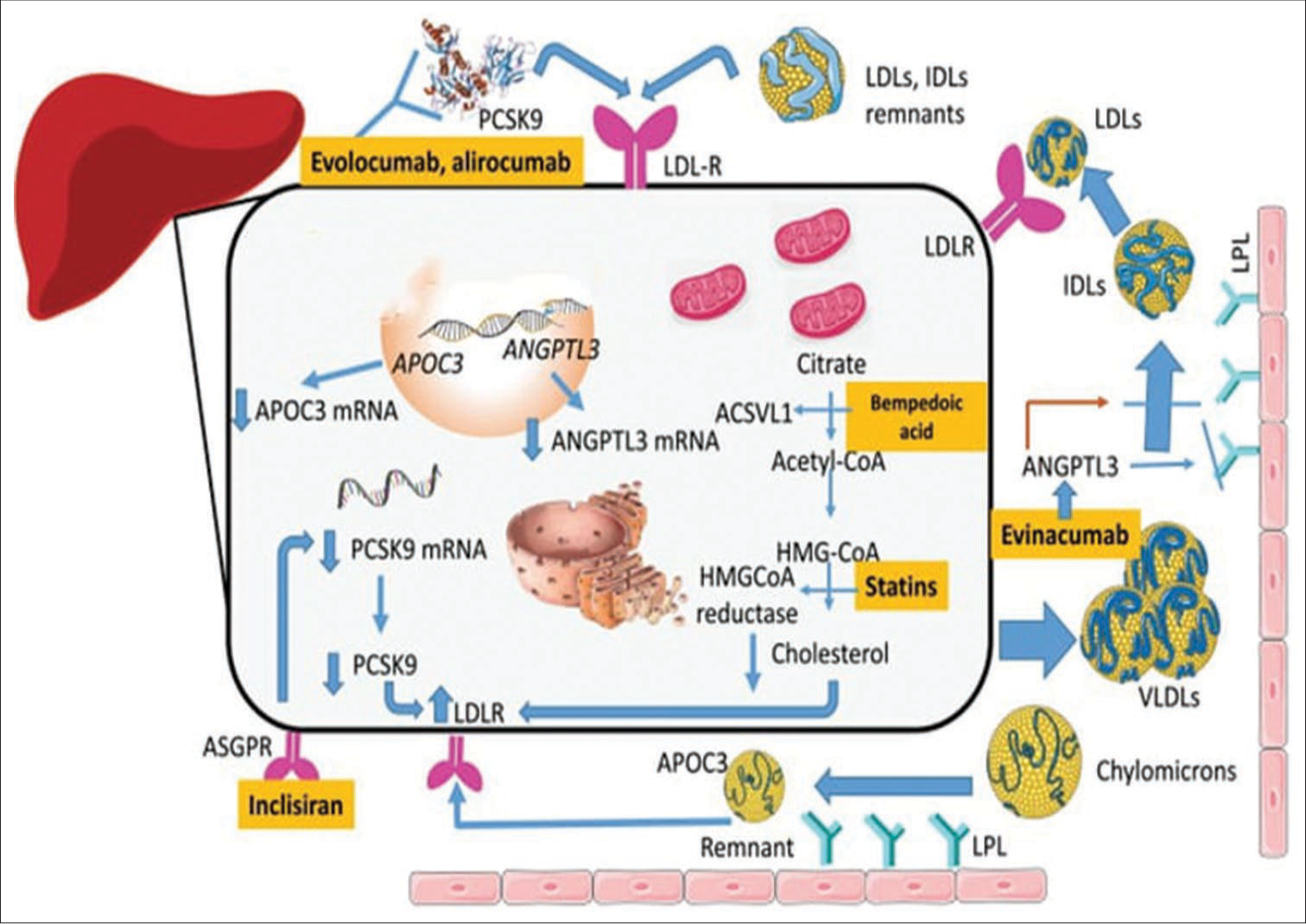
- The methods of action of novel and existing treatments for primary hyperlipidemia. The mainstay of cardiovascular prophylaxis, statins, and target intracellular cholesterol concentrations. Reduced production of cholesterol leads to an increase in the amount of LDL-R in the membrane. The acyl-coenzyme A synthease-1 inhibitor bempedoic acid targets the same metabolic pathway, although it does so at a different stage. In addition, treatments based on PCSK9 modify this pathway by preventing LDLR from being destroyed by lysosomes. A short interfering RNA called inclisiran binds to the ASGPR and is directed against the PCSK9 mRNA. The drug’s tissue selectivity is attributed to the last characteristic, which is the high abundance of ASGPR in the liver – the organ where PCSK9 is mostly produced. The inclisiran-ASGPR complex is endocyted. After entering the cytoplasm, inclisiran interacts with PCSK9 mRNA through the antisense strand, which causes the mRNA to be eliminated by enzymes. An important inhibitor of LPL action is ANGPTL3. Anti-ANGPTL3 treatments enhance the clearance of plasma particles high in triglycerides. Evinacumab, a monoclonal antibody. Triglyceride-rich lipoprotein is recognized by its binding receptors through modulation of recognition by apolipoprotein CIII, which also reduces LPL activity. ANGPTL: Angiopoietin-like protein 3; ACSVL1: Acyl-coenzyme A synthease-1, APOC3: Apolipoprotein C-III, ASGPR: Asialoglycoprotein receptor, HMGCoA reductase: Hydroxymethylglutaryl coenzyme A reductase, IDLs, LDLs, LDLR, LPL: Lipoprotein lipase, PCSK9: Proprotein convertase subtilisinkexin type 9, and VLDLs: lipoproteins with a very low density are used to describe different types of lipoprotein lipase, mRNA: messenger RNA, LDLs: Low-density lipoproteins, LDL-R: Low-density lipoprotein receptor.
Unlike statins, BA and its active metabolite do not utilize the cytochrome P450 pathway. However, BA does decrease organic anion transporter polypeptides, leading to increased creatinine and uric acid levels. Pharmacological interactions primarily occur with simvastatin and pravastatin, necessitating dose adjustments to avoid statin toxicity.[46] BA does not need different dosages for persons with kidney or liver damage, although conclusive studies in advanced kidney failure are lacking. In addition, caution is advised in people who have previously hyperuricemia and gout due to potential increases in uric acid levels.[47]
Notably, tendon injuries and ruptures have been sporadically observed with BA treatment, necessitating discontinuation on detection of inflammation or tendon rupture. BA is available orally at a dose of 180 mg/day, either as a mono drug or combined with ezetimibe.[48] Ongoing trials are investigating BA combined with maximum tolerable statin doses, particularly in secondary prevention and FH. The CLEAR outcomes study aims to evaluate whether BA’s effects on surrogate markers like LDL-C translate into clinical event reduction.
Similarly, inclisiran, an approved small interfering RNA (siRNA) targeting PCSK9 messenger RNA (mRNA), exhibits long-acting effects and has been approved in the European Union for various dyslipidemias. Administered subcutaneously, inclisiran’s mechanism involves its communication with the asialoglycoprotein receptor (ASGPR) in the liver, leading to PCSK9 mRNA degradation.[49] Noteworthy, reductions in LDL-C have been observed in clinical trials, with ongoing studies exploring effectiveness and security in various high-risk populations.
In summary, these emerging therapies offer promising alternatives for dyslipidemia management, with ongoing research shedding light on their effectiveness and security profiles in diverse patient populations.[50]
Recently, novel therapeutic alternatives have emerged targeting complementary metabolic pathways to statin-induced inhibition of hydroxymethyl glutaryl coenzyme A reductase.[51] Among these drugs, BA, and inclisiran, have received FDA approval for a number of indications.
BA functions by preventing ATP-citrate lyase, an essential enzyme in the cholesterol synthesis pathway. It has approval for application in adult patients suffering with HeFH or confirmed cardiovascular atherosclerosis who have not reached their LDL-C treatment goals. Unlike statins, BA is orally administered and has a moderate effect on LDL-C and CRP, an inflammation biomarker.[52] BA’s mechanism of action allows for increased expression of LDLRs on hepatocyte membranes, leading to LDL-C reduction. Notably, BA is a prodrug converted to its active form, BA coenzyme, primarily in the liver and kidney, minimizing myopathic adverse events.
Inclisiran, on the other hand, inhibits PCSK9 use of short interfering RNA for expression (siRNA) targeted against PCSK9 mRNA. This results in reduced intracellular PCSK9 synthesis and increased LDLR expression, leading to LDL-C lowering. Its long-acting effect allows for biannual dosing and has shown a significant reduction in LDL-C levels in clinical trials, particularly in high-risk populations, including FH patients.
In summary, these innovative treatment alternatives provide patients with encouraging substitutes for primary dyslipidemia, FH, and cardiovascular disease, taking care of unmet needs and providing additional benefits beyond LDL-C reduction. Ongoing clinical trials aim to further evaluate their both effectiveness and safety profiles in various patient populations, offering hope for improved management of lipid disorders and cardiovascular risk.[53]
PHARMACOTHERAPY OF EVOLOCUMAB
Evolocumab is a monoclonal antibody indicated for treating various forms of hyperlipidemia and for the prevention of cardiovascular events. For patients with primary hyperlipidemia, including HeFH, it significantly reduces LDL-C, total cholesterol, apolipoprotein B, and non-HDL cholesterol in adults and adolescents aged 12 years and older. For HoFH, evolocumab is recommended for patients aged 13 and older as an adjunct to other lipid-lowering therapies.[54] In addition, it helps reduce the risk of myocardial infarction, stroke, and coronary revascularization in adults with established cardiovascular disease. The dosing regimen for primary hyperlipidemia is 140 mg every 2 weeks or 420 mg once a month, while for HoFH, the dose is 420 mg once a month, administered subcutaneously. Proper storage and handling involve refrigerating the medication and bringing it to room temperature before injection.
Evolocumab works by binding to PCSK9 and preventing it from degrading LDLRs on liver cells, thereby enhancing the clearance of LDL-C from the blood.[55] Its pharmacokinetics includes peak plasma concentrations reached 3–4 days post-administration, a volume of distribution of 3.3–3.9 L, and a half-life of approximately 11–17 days. Clinical trials have demonstrated significant efficacy in lowering LDL-C levels and reducing cardiovascular events. Common side effects include nasopharyngitis, upper respiratory tract infections, influenza, back pain, and injection site reactions, while rare serious effects may involve hypersensitivity and neurocognitive events. Monitoring LDL-C levels and being vigilant for adverse reactions are essential for safe and effective use. Evolocumab is particularly valuable for patients who cannot achieve target LDL-C levels with statins alone or those who are statin-intolerant, representing a significant advancement in lipid management.[56]
PHARMACOTHERAPY OF ALIROCUMAB
Alirocumab is a highly effective PCSK9 inhibitor used to manage primary hyperlipidemia, including HeFH, and to prevent cardiovascular events in adults with established cardiovascular disease.[57] Administered subcutaneously, the recommended starting dose is 75 mg every 2 weeks, which can be increased to 150 mg every 2 weeks if additional LDL-C reduction is necessary. Alternatively, a dosage of 300 mg once every 4 weeks can be used. By inhibiting PCSK9’s interaction with LDLRs, alirocumab prevents their degradation, resulting in increased receptor availability and enhanced clearance of LDL-C from the blood.[58] Clinical trials have demonstrated its ability to significantly lower LDL-C levels and reduce the incidence of cardiovascular events, making it a vital option for patients who do not reach target LDL-C levels with statins alone or those who are statin-intolerant.
Alirocumab reaches peak plasma concentrations within 3–7 days after subcutaneous administration, has a volume of distribution of 0.04–0.05 L/kg, and a half-life of 17–20 days. Common adverse effects include injection site reactions, nasopharyngitis, and flu-like symptoms, while rare side effects may involve upper respiratory tract infections, myalgia, and neurocognitive events. It is contraindicated in patients with hypersensitivity to the drug or its components.[59] Regular monitoring of LDL-C levels and vigilance for side effects are crucial during treatment, especially in the initial phases. Although no dose adjustment is required based on age, caution is advised for pregnant women and pediatric patients due to limited data on safety and efficacy in these populations.
PHARMACOTHERAPY OF EVINACUMAB
Evinacumab is a monoclonal antibody indicated for treating HoFH in patients aged 12 and older.[60] This severe genetic disorder results in extremely high levels of LDL-C due to a defect in the LDLR, leading to poor clearance of LDL-C from the blood. Evinacumab works by inhibiting ANGPTL3, which, in turn, enhances the activity of LPL and endothelial lipase – key enzymes involved in lipid metabolism.[61] This inhibition promotes the clearance of triglycerides and LDL-C from the bloodstream. Administered as an intravenous infusion at a recommended dose of 15 mg/kg every 4 weeks, evinacumab significantly reduces LDL-C levels, as evidenced by clinical trials like the ELIPSE HoFH study,[62] where patients experienced a 49% reduction in LDL-C levels compared to a 3% increase in the placebo group.
Common side effects of evinacumab include infusion-related reactions (such as fever, chills, and rash), upper respiratory tract infections, nasopharyngitis, dizziness, flu-like symptoms, and fatigue. It is contraindicated in patients with known hypersensitivity to the drug or its components. During treatment, patients should be closely monitored for lipid levels, liver and renal function, and potential infusion-related reactions. No significant drug interactions have been identified, but it is important for patients to inform their health-care providers about all medications they are taking. Evinacumab represents a significant advancement for HoFH patients who have not achieved sufficient LDL-C reduction with conventional therapies, offering a novel approach to lowering LDL-C and triglycerides effectively.
CONCLUSION
Novel lipid-lowering medications have been approved by authorities such as the FDA and the European Medicines Agency, primarily for addressing primary hyperlipidemias and cases of secondary prevention. Inclisiran and BA are poised to address significant treatment hurdles for primary hypercholesterolemia, particularly related to short-term adherence and side effects. Positive outcomes from ongoing outcome-based trials are expected to bolster their incorporation into clinical guidelines. In addition, therapies targeting ANGPTL3 show promise in reducing LDL production in the absence of functional LDLRs. However, their role in treating HoFH will require further investigation through long-term add-on studies, potentially combined with plasmapheresis.
Conversely, the inclusion of Evinacumab in clinical guidelines may face challenges. While preventing recurrent pancreatitis would be a significant benefit, larger and longer studies will be necessary to fully establish their efficacy. Primary chylomicronemia presents a critical need for effective therapeutic options, with ANGPTL3-based therapies showing promise. However, their utility may be limited to adult populations as they require some degree of LPL activity to be effective. Other potential indications, such as type 2 diabetes, FPLD, or fatty liver disease, are presently under evaluation or will be explored in the future.
Addressing safety and cost concerns will be crucial in considering the extended use of ApoC-III or ANGPTL3-based medications. The field of primary hyperlipidemia remains dynamic, with ongoing research and innovation driving continuous evolution in treatment strategies as new medications demonstrate reasonable cost-effectiveness ratios, they contribute to advancing the management of primary hyperlipidemia.
Furthermore, evolocumab, alirocumab, and evinacumab are pivotal in managing hypercholesterolemia, especially for patients with genetic conditions such as HoFH and HoFH, or those who cannot meet LDL-C targets with statins alone. These monoclonal antibodies use distinct mechanisms to effectively reduce LDL-C levels and decrease cardiovascular risks. Consistent monitoring and attention to possible side effects are crucial for maximizing the benefits and ensuring the safety of these treatments.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Practical definitions of severe versus familial hypercholesterolaemia and hypertriglyceridaemia for adult clinical practice. Lancet Diabetes Endocrinol. 2019;7:880-6.
- [CrossRef] [PubMed] [Google Scholar]
- Familial hypertriglyceridemia/polygenic hypertrigliceridemia. Clin Investig Arterioscler. 2021;33(Suppl 2):37-42.
- [CrossRef] [PubMed] [Google Scholar]
- Rare treatments for rare dyslipidemias: New perspectives in the treatment of homozygous familial hypercholesterolemia (HoFH) and familial chylomicronemia syndrome (FCS) Curr Atheroscler Rep. 2021;23:65.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term risk of atherosclerotic cardiovascular disease in US adults with the familial hypercholesterolemia phenotype. Circulation. 2016;134:9-19.
- [CrossRef] [PubMed] [Google Scholar]
- Detecting familial hypercholesterolemia earlier in life by actively searching for affected children: The DECOPIN project. Atherosclerosis. 2018;278:210-6.
- [CrossRef] [PubMed] [Google Scholar]
- LDL cholesterol: Lower, faster, younger? Lancet Diabetes Endocrinol. 2020;8:5-7.
- [CrossRef] [PubMed] [Google Scholar]
- Knowns and unknowns in the care of pediatric familial hypercholesterolemia. J Lipid Res. 2017;58:1765-76.
- [CrossRef] [PubMed] [Google Scholar]
- Why patients with familial hypercholesterolemia are at high cardiovascular risk? Beyond LDL-C levels. Trends Cardiovasc Med. 2021;31:205-15.
- [CrossRef] [PubMed] [Google Scholar]
- A comprehensive update on the chylomicronemia syndrome. Front Endocrinol (Lausanne). 2020;11:593931.
- [CrossRef] [PubMed] [Google Scholar]
- The burden of familial chylomicronemia syndrome in Canadian patients. Lipids Health Dis. 2020;19:120.
- [CrossRef] [PubMed] [Google Scholar]
- The association of triglyceride levels with the incidence of initial and recurrent acute pancreatitis. Lipids Health Dis. 2021;20:72.
- [CrossRef] [PubMed] [Google Scholar]
- Familial hypercholesterolaemia: Evolving knowledge for designing adaptive models of care. Nat Rev Cardiol. 2020;17:360-77.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of a multidisciplinary lipid clinic to improve the care of individuals with severe lipid conditions: A RE-AIM framework analysis. Implement Sci Commun. 2021;2:32.
- [CrossRef] [PubMed] [Google Scholar]
- Does lifestyle contribute to disease severity in patients with inherited lipid disorders? Curr Opin Lipidol. 2017;28:177-85.
- [CrossRef] [PubMed] [Google Scholar]
- 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: Part 1, lifestyle and behavioral factors. JAMA Cardiol. 2019;4:1043-4.
- [CrossRef] [PubMed] [Google Scholar]
- Familial chylomicronemia syndrome: Bringing to life dietary recommendations throughout the life span. J Clin Lipidol. 2018;12:908-19.
- [CrossRef] [PubMed] [Google Scholar]
- Use of lifestyle modifications for management of a patient with severely high total cholesterol (> 14 mmol/L) and triglycerides (> 40 mmol/L) J Lifestyle Med. 2021;11:43-6.
- [CrossRef] [PubMed] [Google Scholar]
- Acute pancreatitis secondary to oral contraceptive-induced hypertriglyceridemia: A case report. Gynecol Endocrinol. 2018;34:930-2.
- [CrossRef] [PubMed] [Google Scholar]
- Detection and management of familial hypercholesterolaemia in primary care in Australia: Protocol for a pragmatic cluster intervention study with pre-post intervention comparisons. BMJ Open. 2017;7:e017539.
- [CrossRef] [PubMed] [Google Scholar]
- Familial dysbetalipoproteinemia: An underdiagnosed lipid disorder. Curr Opin Endocrinol Diabetes Obes. 2017;24:133-9.
- [CrossRef] [PubMed] [Google Scholar]
- 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111-88.
- [CrossRef] [PubMed] [Google Scholar]
- Rare dyslipidaemias, from phenotype to genotype to management: A European atherosclerosis society task force consensus statement. Lancet Diabetes Endocrinol. 2020;8:50-67.
- [CrossRef] [PubMed] [Google Scholar]
- Consensus statement by the American association of clinical endocrinologists and American college of endocrinology on the management of dyslipidemia and prevention of cardiovascular disease algorithm-2020 executive summary. Endocr Pract. 2020;26:1196-224.
- [CrossRef] [PubMed] [Google Scholar]
- Nonadherence to statins: Individualized intervention strategies outside the pill box. Vasc Health Risk Manag. 2018;14:91-102.
- [CrossRef] [PubMed] [Google Scholar]
- Feasibility of improving identification of familial hypercholesterolaemia in general practice: Intervention development study. BMJ Open. 2016;6:e011734.
- [CrossRef] [PubMed] [Google Scholar]
- 2021 Canadian cardiovascular society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in adults. Can J Cardiol. 2021;37:1129-50.
- [CrossRef] [PubMed] [Google Scholar]
- Lipid management in patients with endocrine disorders: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2020;105:dgaa674.
- [CrossRef] [PubMed] [Google Scholar]
- Lipid therapy: A new whiteboard video for patient education. Can Pharm J (Ott). 2021;154:175-8.
- [CrossRef] [PubMed] [Google Scholar]
- Practical guidance for combination lipid-modifying therapy in high-and very-high-risk patients: A statement from a European atherosclerosis society task force. Atherosclerosis. 2021;325:99-109.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of adding bezafibrate to standard lipid-lowering therapy on post-fat load lipid levels in patients with familial dysbetalipoproteinemia: A randomized placebo-controlled crossover trial. J Lipid Res. 2017;58:2180-7.
- [CrossRef] [PubMed] [Google Scholar]
- Challenges in the care of familial hypercholesterolemia: A community care perspective. Expert Rev Cardiovasc Ther. 2015;13:1091-100.
- [CrossRef] [PubMed] [Google Scholar]
- Differentiating familial chylomicronemia syndrome from multifactorial severe hypertriglyceridemia by clinical profiles. J Endocr Soc. 2019;3:2397-410.
- [CrossRef] [PubMed] [Google Scholar]
- Polygenic hyperlipidemias and coronary artery disease risk. Circ Genom Precis Med. 2020;13:e002725.
- [CrossRef] [PubMed] [Google Scholar]
- Barriers, facilitators, and solutions to familial hypercholesterolemia treatment. PLoS One. 2020;15:e0244193.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of LDL-lowering therapy among men and women: Meta-analysis of individual data from 174, 000 participants in 27 randomised trials. Lancet. 2015;385:1397-405.
- [CrossRef] [PubMed] [Google Scholar]
- Lipid-modifying therapy and low-density lipoprotein cholesterol goal attainment in patients with familial hypercholesterolemia in Germany: The CaReHigh registry. Atherosclerosis. 2018;277:314-22.
- [CrossRef] [PubMed] [Google Scholar]
- Pharmacological lipid-modification therapies for prevention of ischaemic heart disease: Current and future options. Lancet. 2019;394:697-708.
- [CrossRef] [PubMed] [Google Scholar]
- A meta-analysis of medications directed against PCSK9 in familial hypercholesterolemia. Atherosclerosis. 2021;325:46-56.
- [CrossRef] [PubMed] [Google Scholar]
- Therapeutic response to medium-chain triglycerides and omega-3 fatty acids in a patient with the familial chylomicronemia syndrome. Arterioscler Thromb Vasc Biol. 1997;17:1400-6.
- [CrossRef] [PubMed] [Google Scholar]
- PPAR control of metabolism and cardiovascular functions. Nat Rev Cardiol. 2021;18:809-23.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of TPE vs medical management on patient outcomes in the setting of hypertriglyceridemia-induced acute pancreatitis with severely elevated triglycerides. J Clin Apher. 2021;36:719-26.
- [CrossRef] [PubMed] [Google Scholar]
- Familial chylomicronemia syndrome: A clinical guide for endocrinologists. Endocr Pract. 2018;24:756-63.
- [CrossRef] [PubMed] [Google Scholar]
- Recent developments in pharmacotherapy for hypertriglyceridemia: What's the current state of the art? Expert Opin Pharmacother. 2020;21:107-20.
- [CrossRef] [PubMed] [Google Scholar]
- Role of bempedoic acid in clinical practice. Cardiovasc Drugs Ther. 2021;35:853-64.
- [CrossRef] [PubMed] [Google Scholar]
- Liver-specific ATP-citrate lyase inhibition by bempedoic acid decreases LDL-C and attenuates atherosclerosis. Nat Commun. 2016;7:13457.
- [CrossRef] [PubMed] [Google Scholar]
- Role of diet in hyperuricemia and gout. Best Pract Res Clin Rheumatol. 2021;35:101723.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): A randomised, open-label, non-inferiority trial. Lancet. 2022;400:380-90.
- [CrossRef] [PubMed] [Google Scholar]
- Proprotein convertase subtilisin/Kexin 9 (PCSK9) promotes macrophage activation via LDL receptor-independent mechanisms. Circ Res. 2022;131:873-89.
- [CrossRef] [PubMed] [Google Scholar]
- Dyslipidemia management in 2020: An update on diagnosis and therapeutic perspectives. Endocr Metab Immune Disord Drug Targets. 2021;21:815-34.
- [CrossRef] [PubMed] [Google Scholar]
- Statin-induced myopathy is associated with mitochondrial complex III inhibition. Cell Metab. 2015;22:399-407.
- [CrossRef] [PubMed] [Google Scholar]
- Tirzepatide therapy in a patient with type 2 diabetes mellitus, chylomicronemia, and heterozygosity for lipoprotein lipase deficiency. AACE Clin Case Rep. 2023;9:128-30.
- [CrossRef] [PubMed] [Google Scholar]
- 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111-88.
- [CrossRef] [PubMed] [Google Scholar]
- Pleiotropic effects of PCSK-9 inhibitors. Int J Mol Sci. 2021;22:3144.
- [CrossRef] [PubMed] [Google Scholar]
- Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-22.
- [CrossRef] [PubMed] [Google Scholar]
- Familial hypercholesterolemia and its current diagnostics and treatment possibilities: A literature analysis. Medicina (Kaunas). 2022;58:1665.
- [CrossRef] [PubMed] [Google Scholar]
- Transiently achieved very low LDL-cholesterol levels by statin and alirocumab after acute coronary syndrome are associated with cardiovascular risk reduction: The ODYSSEY OUTCOMES trial. Eur Heart J. 2023;44:1408-17.
- [CrossRef] [PubMed] [Google Scholar]
- Alirocumab for the treatment of hypercholesterolemia. Expert Opin Biol Ther. 2017;17:633-43.
- [CrossRef] [PubMed] [Google Scholar]
- Evinacumab for homozygous familial hypercholesterolemia. N Engl J Med. 2020;383:711-20.
- [CrossRef] [PubMed] [Google Scholar]
- Angiopoietin-like protein 3 (ANGPTL3) inhibitors in the management of refractory hypercholesterolemia. Clin Pharmacol. 2022;14:49-59.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of homozygous familial hypercholesterolemia with ANGPTL3 inhibitor, evinacumab. JCEM Case Rep. 2023;1:luad058.
- [CrossRef] [PubMed] [Google Scholar]






